Resultant Ruisidilasu from generic delavird and resveratrol, and synthetic method
A technology of delavirdine and resveratrol, applied in the field of chemical pharmacy, can solve problems such as low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
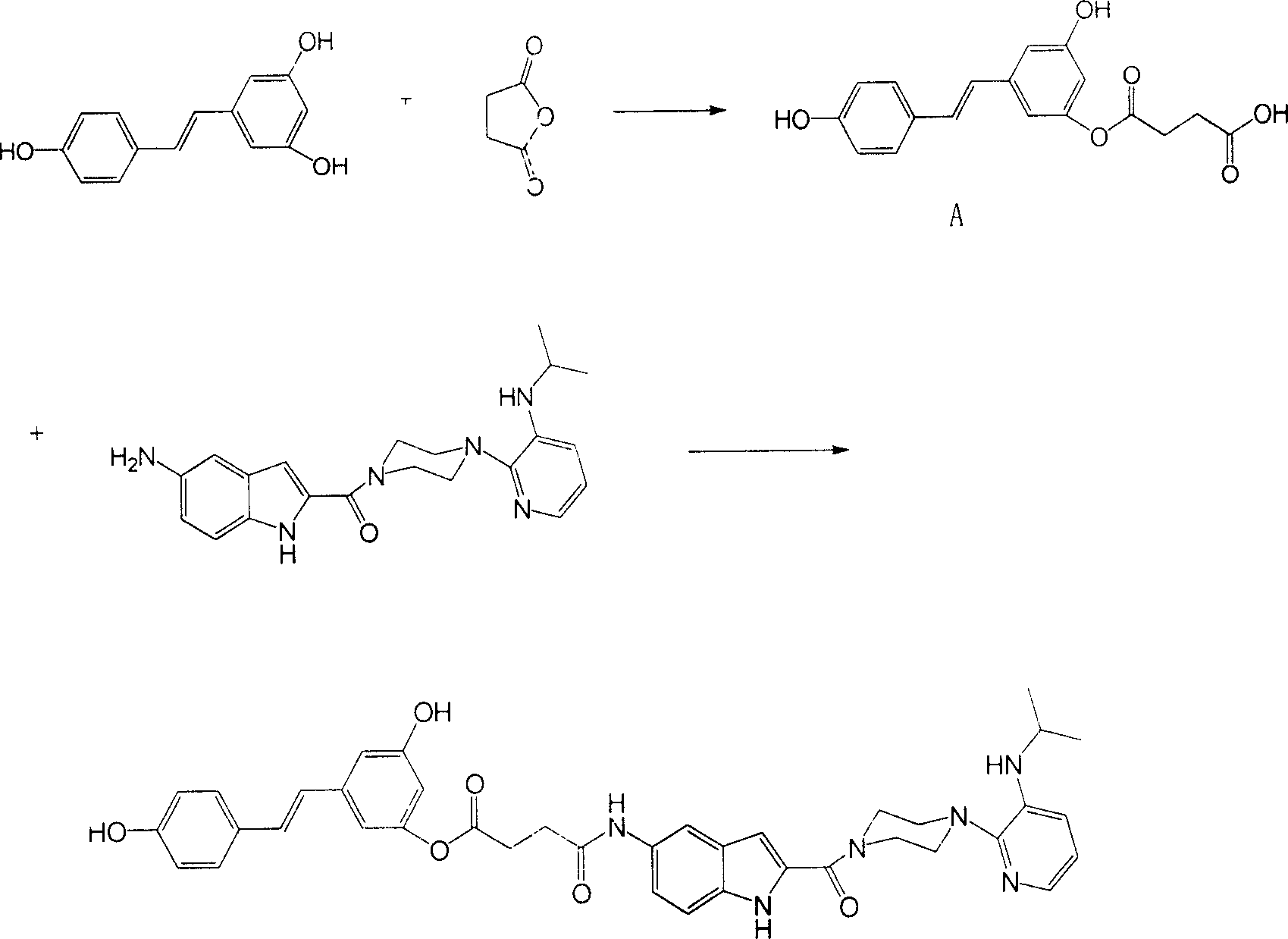
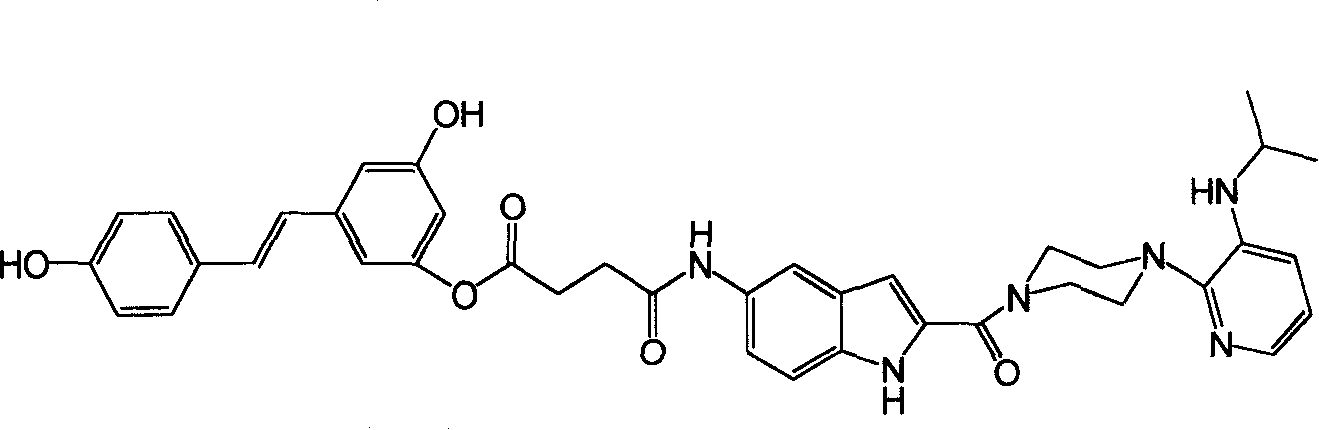
[0032]Take resveratrol 5g (0.022mol), NaOH 2.64g (0.066mol), water 80mL, succinic anhydride 2.2g (0.022mol). Stir the reaction at room temperature for 30 minutes, and acidify the reaction solution with 100 mL of dilute hydrochloric acid (1 mol / L). Extracted with ethyl acetate, anhydrous Na 2 SO 4 dry. The crude product was subjected to silica gel column chromatography (developing solvent: ethyl acetate:petroleum ether:acetic acid=1:1:0.01, v:v) to obtain Compound A. Take compound A 1.47g (4.5mmol), DCC 1.42g (6.9mmol), THF 25mL, delavirdine intermediate 1.3g (3.4mmol). The reaction was stirred at room temperature for 5 h, and DCU was removed by filtration. The crude product was subjected to silica gel column chromatography (developing solvent: chloroform:methanol=20:1, v:v) to obtain 1.1g (46%) of the compound risdelasine such as figure 1 shown.
Embodiment 2
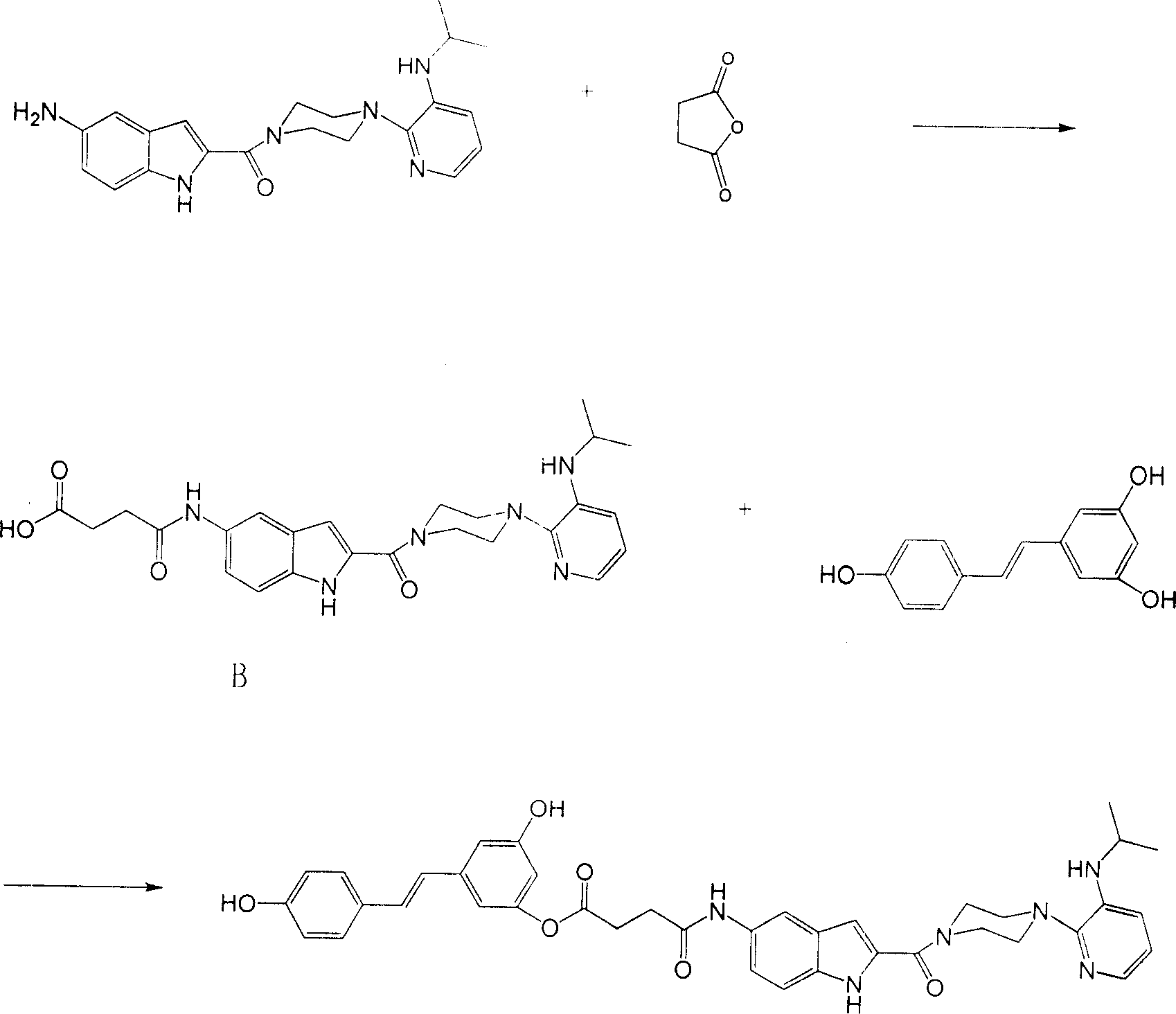
[0034] Take delavirdine 1g (2.65mmol), THF 10mL, succinic anhydride 0.265g (2.65mmol). Stir the reaction at room temperature for 30 min, add 200 mL of diethyl ether to precipitate, filter, and dry to obtain 1.15 mg (91%) of Compound B. Take compound B 1.265g (2.65mmol), DCC 0.57g (2.78mmol), THF 10mL, DMAP 0.1g (0.82mmol), resveratrol 0.63g (2.78mmol). The reaction was stirred at room temperature for 5 h, and DCU was removed by filtration. The crude product was subjected to silica gel column chromatography (developing solvent: chloroform:methanol=20:1, v:v) to obtain the compound risdelatin. Such as figure 2 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com