Monoazo/iron complex compound, charge control agent comprising the same, and toner
A technology of coordination compounds and charge control agents, which is applied to the complex metal compounds of azo dyes, azo dyes, developers, etc., can solve the problems of fire, explosion, drying and crushing process hazards, etc., and achieve the change of charging characteristics Small size, good kneading dispersibility, and high charge-enhancing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
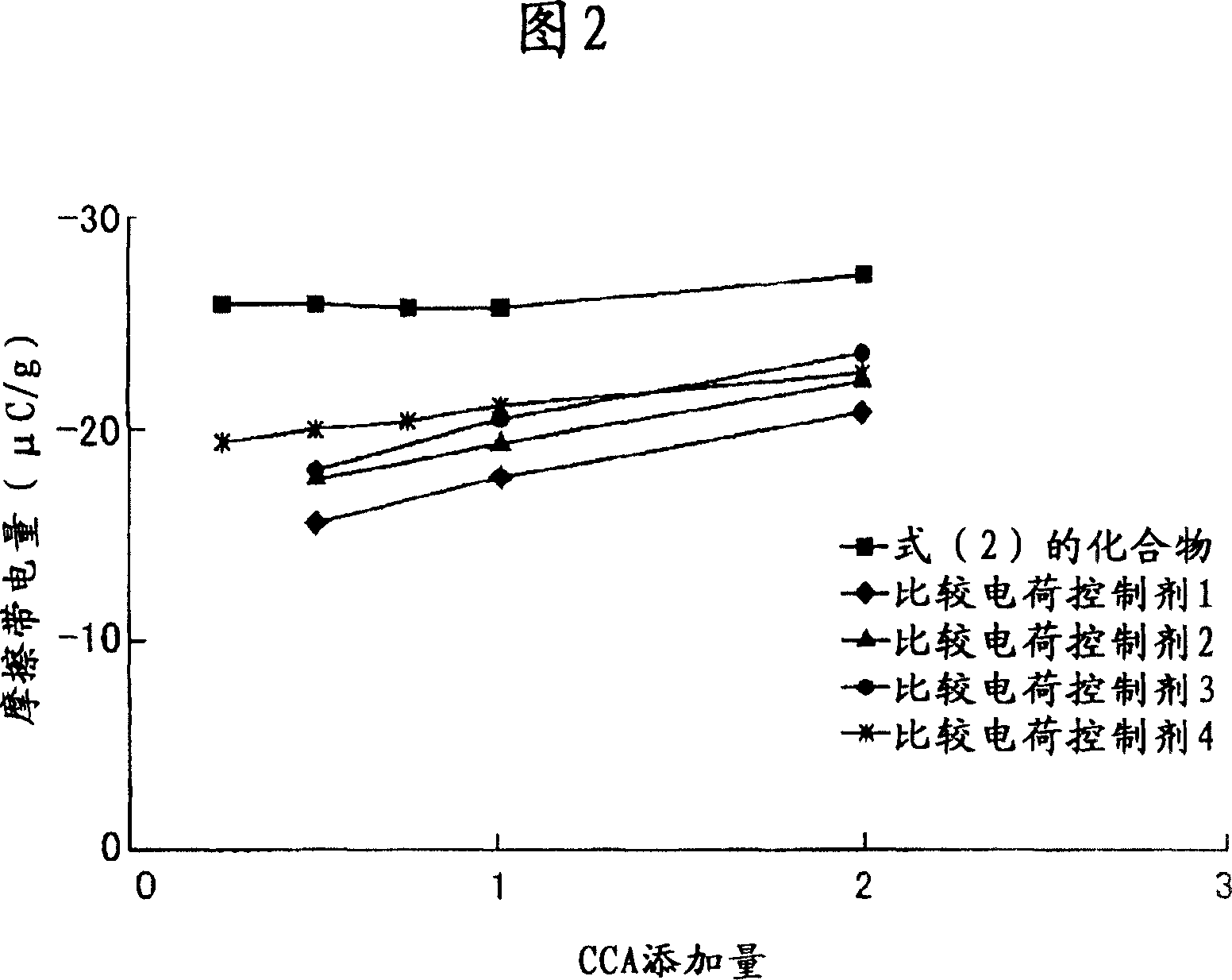
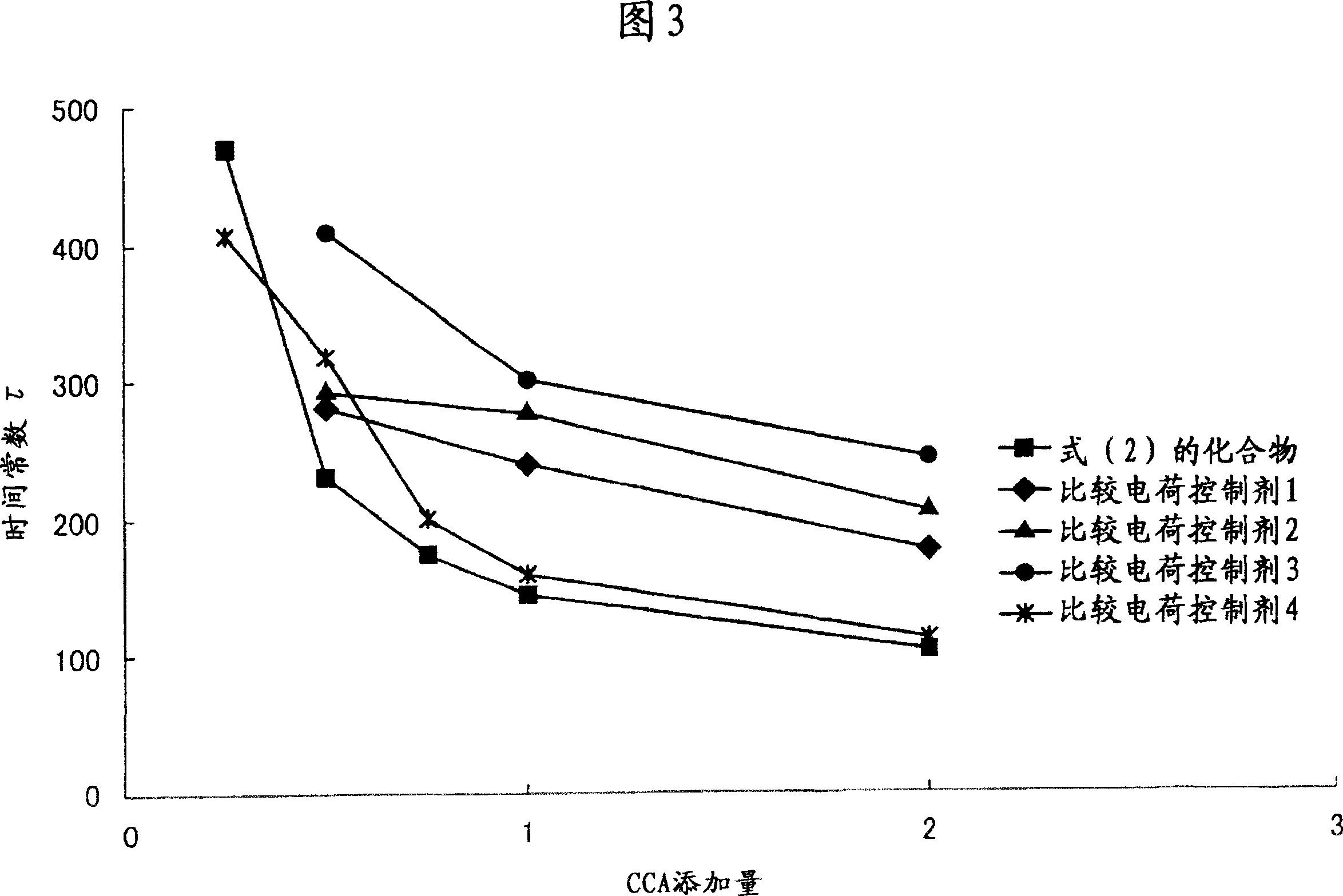
Examples
Embodiment 1
[0187] Hereinafter, although an Example demonstrates this invention, these do not limit the scope of the present invention. In addition, all "parts" in an Example represent "parts by mass".
manufacture example 1
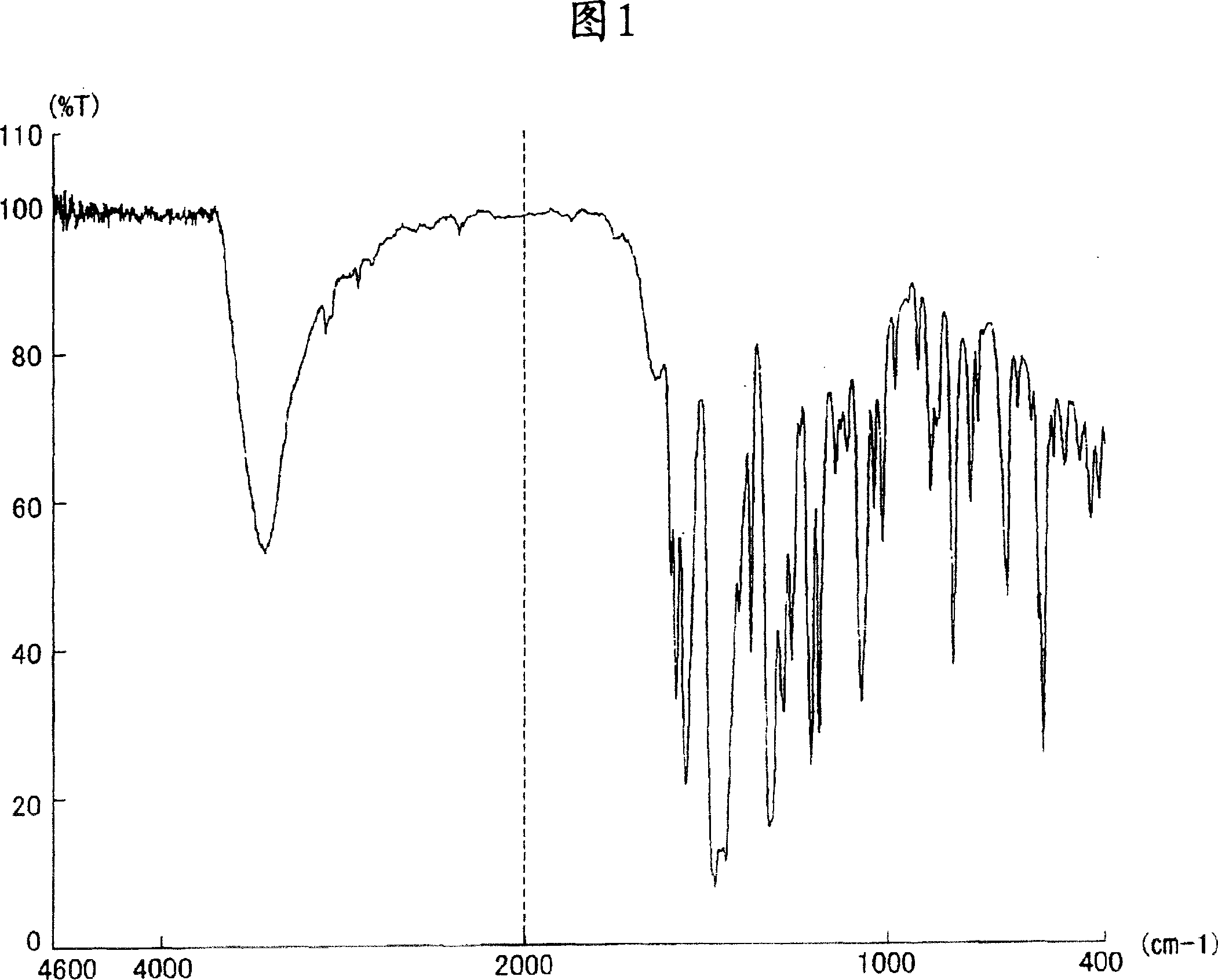
[0188] [Production Example 1] Production of Compound of Formula (2)
[0189] Add 57.4 parts of 4-chloro-2-aminophenol to 580 parts of water and 84 parts of 35% hydrochloric acid, and dissolve with stirring under cooling. At an internal temperature of 10°C or lower, 28.2 parts of sodium nitrite dissolved in 50.7 parts of water was dropped into the above hydrochloric acid aqueous solution, and the temperature was maintained at 5-10°C while adding 50 parts of appropriate crushed ice. After the dropwise addition was terminated, the reaction was stirred at 10° C. for 2 hours. Add 7.3 parts of sulfamic acid, react for 10 minutes, confirm with potassium iodide starch test paper that no excess nitrous acid remains, and prepare a diazo solution.
[0190]Then, 101 parts of 3-methyl-1-(3,4-dichlorophenyl)-5-pyrazolone was added to a mixed solution of 475 parts of water, 95 parts of sodium carbonate and 840 parts of n-butanol, Stir at room temperature to dissolve. Inject the above diaz...
manufacture example 2
[0204] [Production Example 2] Another production example of the compound of formula (2)
[0205] Add 68 parts of 4-chloro-2-aminophenol to 500 parts of water and 147 parts of 35% hydrochloric acid, stir and dissolve under cooling. At an internal temperature of 10°C or lower, 33.4 parts of sodium nitrite dissolved in 61.6 parts of water was dropped into the above hydrochloric acid aqueous solution, and the temperature was maintained at 5-10°C while adding 40 parts of appropriate crushed ice. After the dropwise addition was terminated, the reaction was stirred at 10° C. for 2 hours. Add 6.8 parts of sulfamic acid, react for 10 minutes, confirm with potassium iodide starch test paper that no excess nitrous acid remains, and prepare a diazo solution.
[0206] Then, 117.5 parts of 3-methyl-1-(3,4-dichlorophenyl)-5-pyrazolone was added to a mixed solution of 616 parts of water, 113.6 parts of sodium carbonate and 660 parts of n-butanol, Stir at room temperature to dissolve. Injec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com