Process for preparing P-nitro benzoic acid by bionically catalystically oxidizing P-nitro toluene with oxygen
A technology of p-nitrobenzoic acid and p-nitrotoluene, applied in chemical instruments and methods, preparation of organic compounds, physical/chemical process catalysts, etc., can solve problems such as health threats to industrial operators, and achieve the goal of reducing production costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
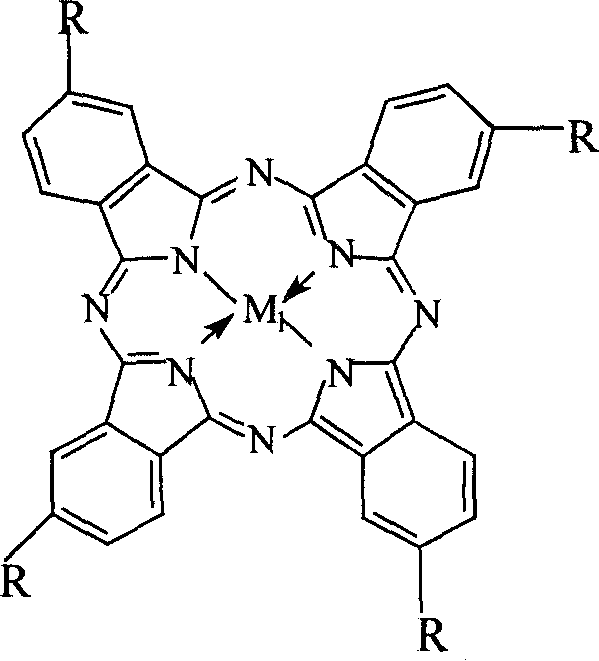
[0022] Get 2mg cobalt phthalocyanine (i.e. R=H in general formula (I), M 1 =Co), 1.4g p-nitrotoluene and 2g sodium hydroxide were packed into a 200mL autoclave, and 38mL of ethanol aqueous solution (95% ethanol: 22mL, water: 16mL) was added containing 55% (V / V) of ethanol, and the Oxygen gas with a pressure of 2.0 MPa was introduced, and the reaction was carried out in a water bath at a temperature of 40° C. for 14 hours. After the reaction, samples were taken, and the reaction mixture was filtered with suction to remove the catalyst, then 15 mL of distilled water was added, neutralized with dilute hydrochloric acid, and filtered. The obtained product was analyzed and detected by high-pressure liquid chromatography, and the yield of p-nitrobenzoic acid was 96.2%.
Embodiment 2
[0024] Get 22mg iron phthalocyanine (i.e. R=H in general formula (I), M 1 =Fe), 6.3g p-nitrotoluene and 9.0g sodium hydroxide were put into a 200mL autoclave, and 38mL of ethanol aqueous solution (95% ethanol: 28mL, water: 10mL) containing 70% (V / V) ethanol was added, The reaction pressure was 3.0 MPa, the temperature was controlled at 35° C. in a water bath, and the reaction was carried out for 8 hours. The aftertreatment steps are the same as in Example 1, and the obtained product is analyzed and detected by high-pressure liquid chromatography, and the yield of p-nitrobenzoic acid is 96.4%.
Embodiment 3
[0026] Get 10mg copper phthalocyanine (i.e. R=H in general formula (I), M 1 =Cu), 1.0g of p-nitrotoluene and 2.5g of sodium hydroxide were charged into a 200mL autoclave, and 38mL of aqueous ethanol (95% ethanol: 30mL, water: 8mL) containing 75% (V / V) of ethanol was added, The oxygen pressure is 2.5MPa, the temperature is controlled at 40°C in a water bath, and the reaction is performed for 10 hours. The aftertreatment steps are the same as in Example 1, and the obtained product is analyzed and detected by high-pressure liquid chromatography, and the yield of p-nitrobenzoic acid is 93.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com