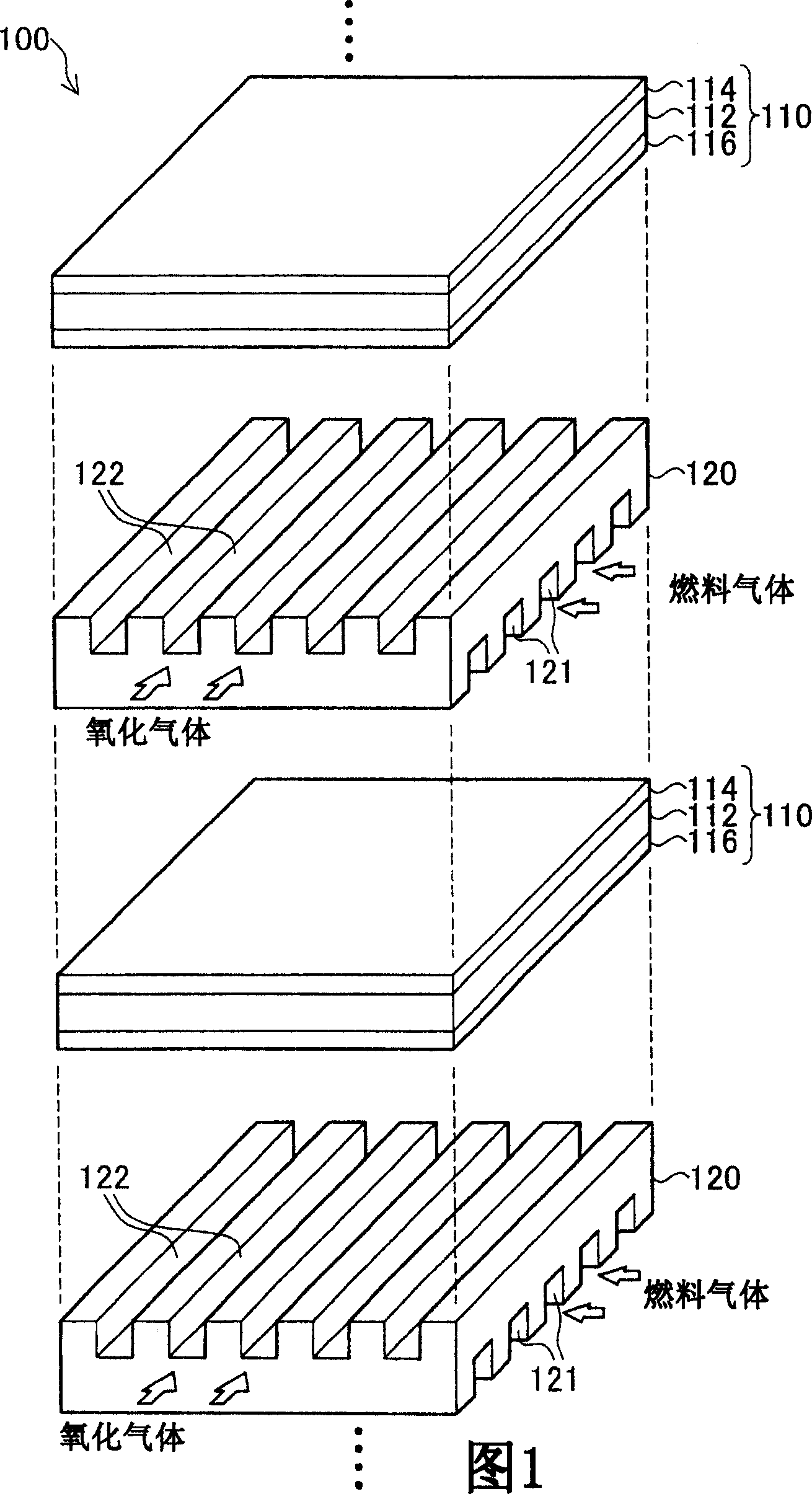
Cathode for fuel cell and process of the same
A fuel cell and cathode technology, which is applied in the direction of fuel cells, fuel cell parts, solid electrolyte fuel cells, etc., can solve the problem that the power generation performance of fuel cells cannot be improved, fuel cells cannot be used, and oxygen ion conductivity and electron conductivity are not good. Sufficient and other issues, to achieve the effect of improving the conductivity of oxygen ions, improving the performance of power generation, and improving the performance of electrons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
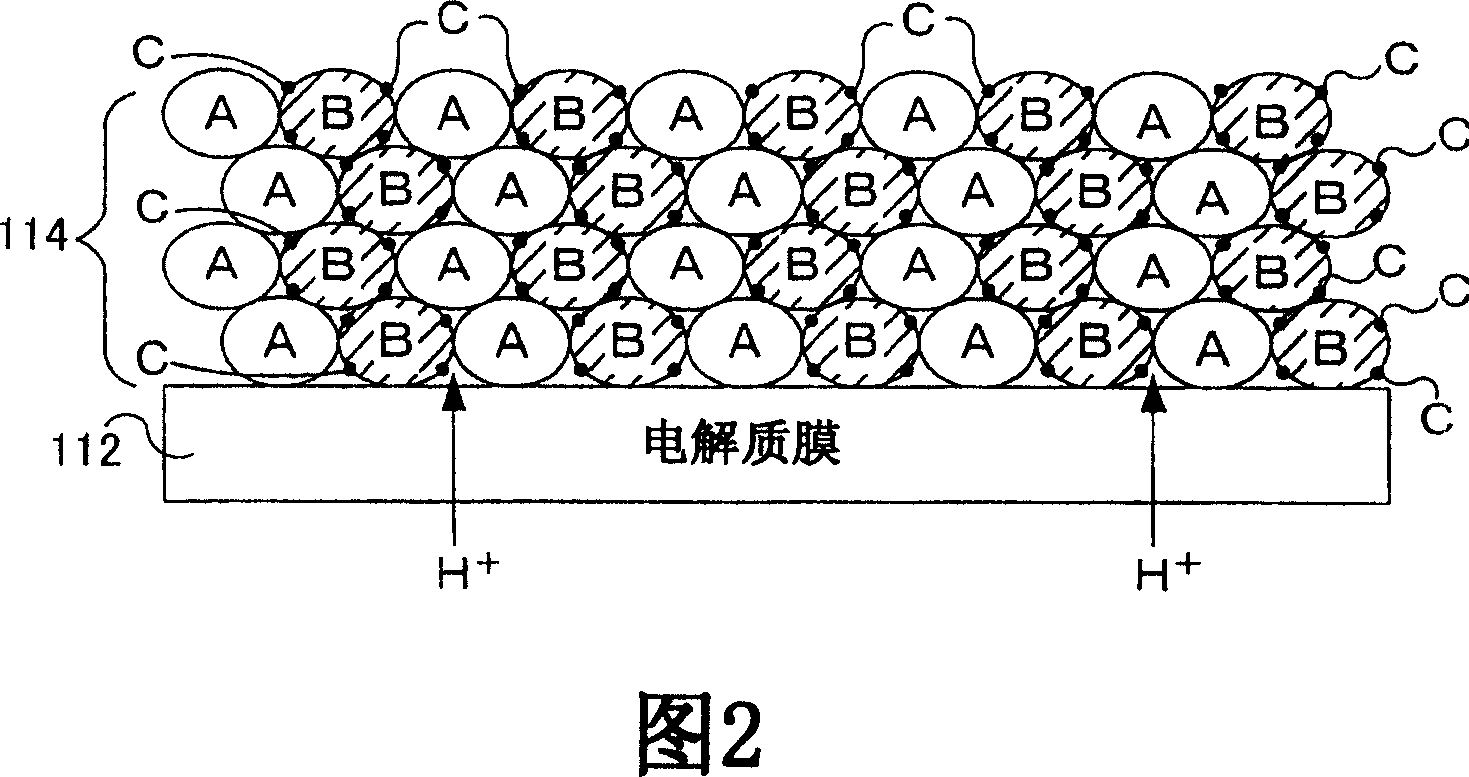
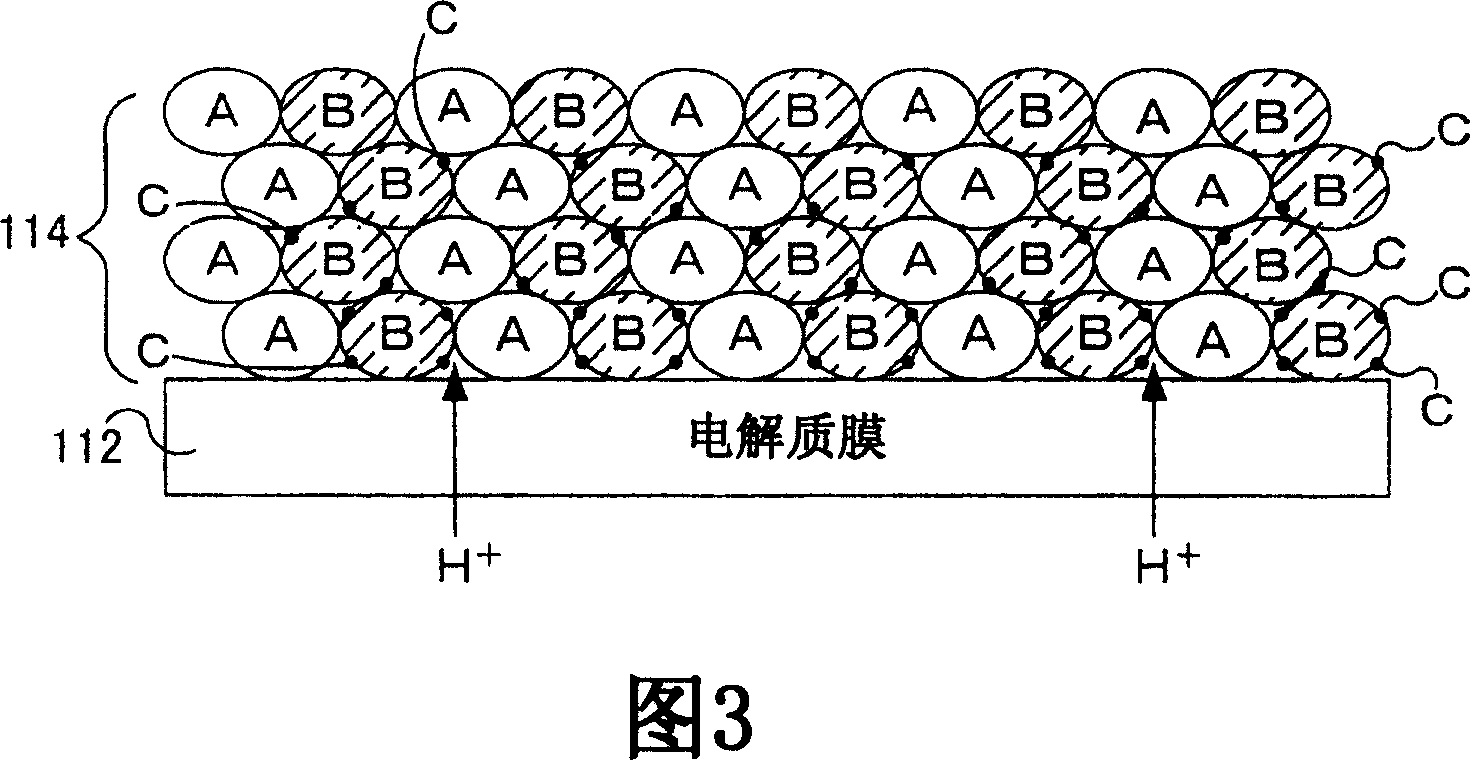
[0070] In Example 1, using Bi 1.5 Dy 0.5 o 3 As composite oxide B, La 0.5 Sr 0.5 MnO 3 As the inorganic material A, as the oxygen dissociation promoting catalyst C, Pt was used. Bi used as composite oxide B 1.5 Dy 0.5 o 3 As shown in Figure 9, it has at least 10 -2 Oxygen ion conductivity (S / cm).
[0071] The oxygen dissociation-promoting catalyst C is first supported on the composite oxide B before being mixed with the inorganic material A as described in the method of manufacturing the cathode 114 described above. Using chloroplatinic acid solution in Bi 1.5 Dy 0.5 o 3 The powder was loaded with 0.5% by weight of Pt. Mix the platinum support Bi in a 1:1 (by volume) ratio using a mortar and pestle 1.5 Dy 0.5o 3 and La 0.5 Sr 0.5 MnO 3 , mix it with a binder and a solvent, and pass the mixture through a roll crusher to obtain a paste. The resulting paste was screen printed onto the electrolyte membrane and dried at 90°C. The paste was screen-printed and dr...
Embodiment 2
[0074] In the example, using La 0.7 Sr 0.3 Ga 0.7 Fe 0.3 o 3 As composite oxide B, La 0.5 Sr 0.5 MnO 3 As the inorganic material A, as the oxygen dissociation promoting catalyst C, Pt was used. La used as composite oxide B 0.7 Sr 0.3 Ga 0.7 Fe 0.3 o 3 As shown in Figure 9, it has at least 10 -2 Oxygen ion conductivity (S / cm).
[0075] In Example 2, the oxygen dissociation-promoting catalyst C is supported after mixing the composite oxide B and the inorganic material A, unlike the above-mentioned method of manufacturing the cathode 114 . Mix La in a 1:1 (by volume) ratio using a mortar and pestle 0.7 Sr 0.3 Ga 0.7 Fe 0.3 o 3 and La 0.5 Sr 0.5 MnO 3 . The obtained mixture powder was immersed in a chloroplatinic acid solution, dried at 80° C., and fired at 500° C. to support 0.5% by weight of Pt. The resulting platinum-loaded mixture (A+B+C) was mixed with binder and solvent and passed through a roll mill to obtain a paste. The resulting paste was screen p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com