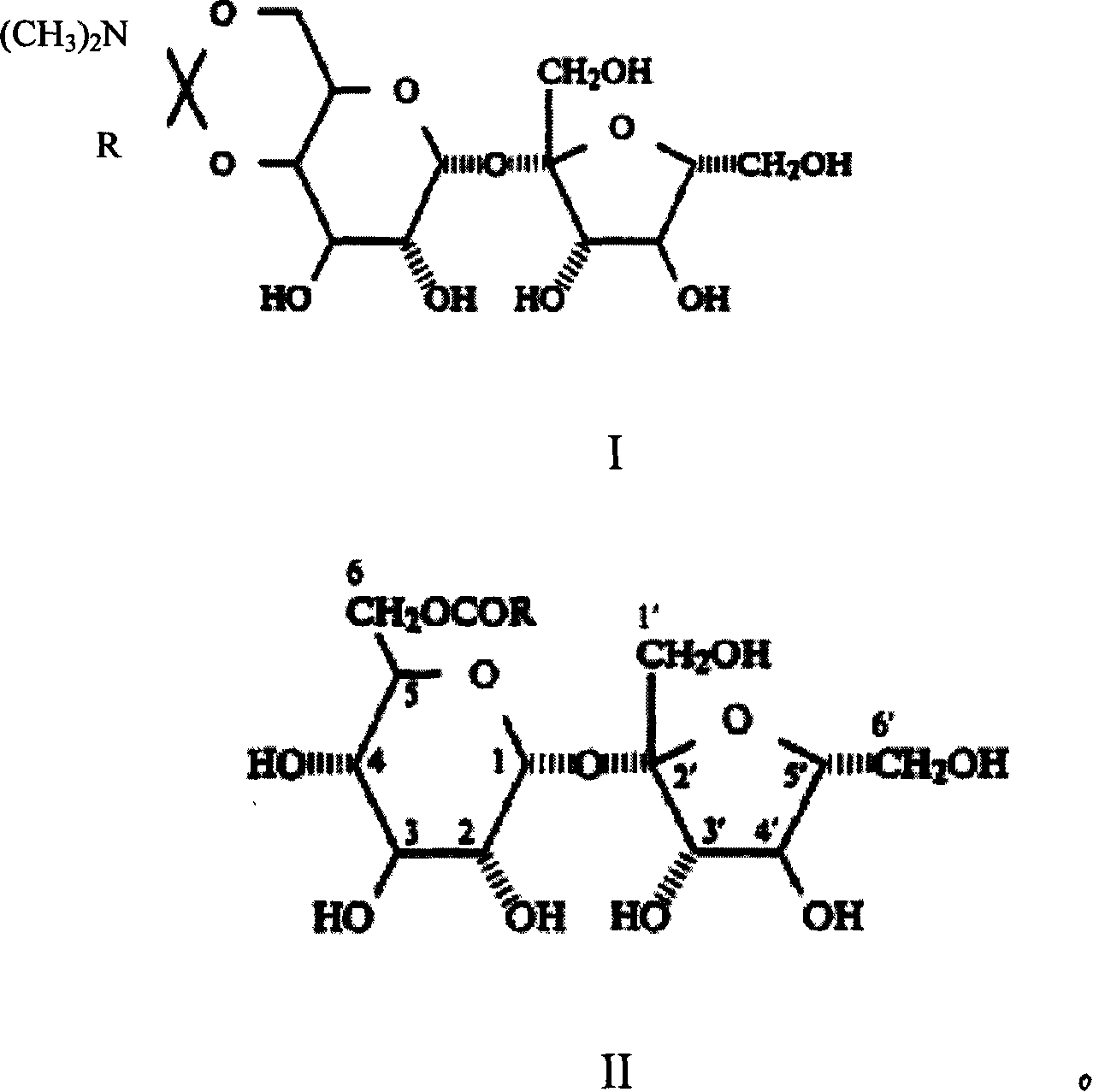
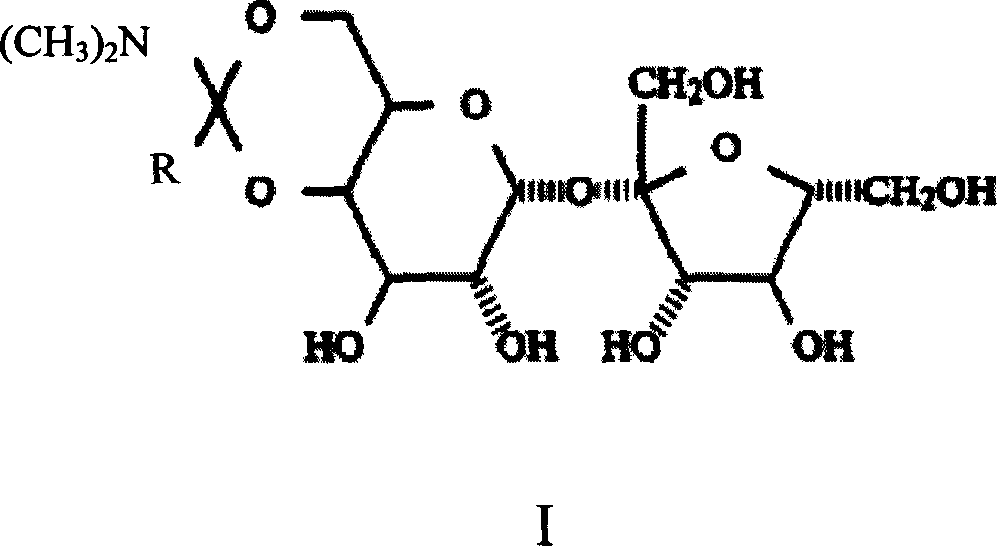
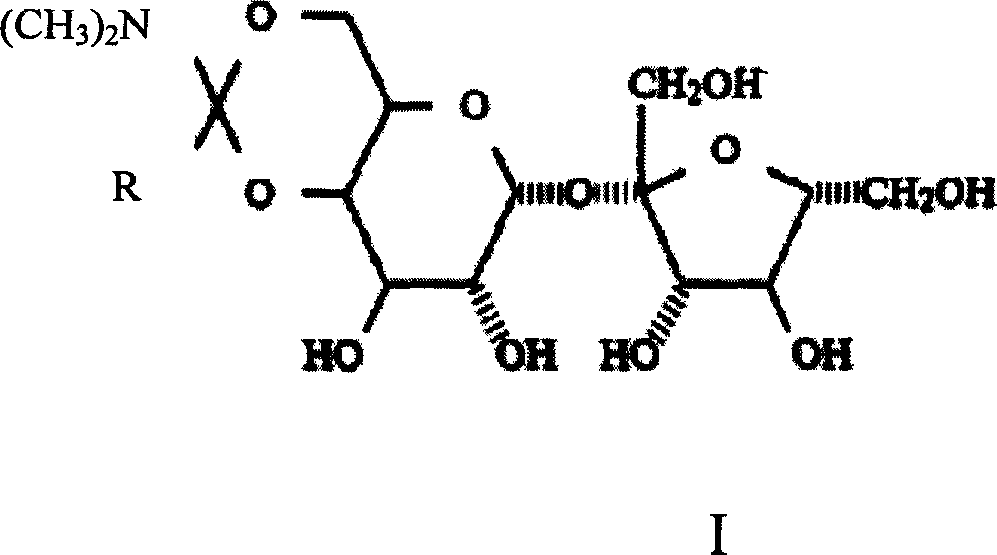
Method of preparing trichloro sucrose-6-organic acid ester
A technology of organic acid esters and sucrose, applied in organic chemistry, preparation of sugar derivatives, chemical instruments and methods, etc., can solve the problems of large investment and complicated process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1, the synthesis of sucrose-6-ethyl ester
[0023] Take 34.2 grams of sucrose, add it to a four-neck flask equipped with stirring, add 140ml of DMF, raise the temperature to 90°C, stir for 30 minutes, after the sucrose is completely dissolved, cool down to 40°C, add 14 grams of N,N-dimethyl Acetamide dimethyl acetal, after the addition, keep warm at 40-50°C for 2.5 hours, remove part of the solvent under reduced pressure, cool down to below 20°C, add 14ml of water, and adjust the pH of the system to about 6.5 with acetic acid , continue stirring for 30 minutes. Stop responding. Liquid chromatography detection showed that the content of sucrose-6-ethyl ester was 28.3%, and the yield was 85%.
Embodiment 2
[0024] Embodiment 2, the synthesis of sucrose-6-ethyl ester
[0025] Take 20 grams of pulverized sucrose, add it to a four-neck flask equipped with stirring, add 50ml DMF and 40ml pyridine, heat up to 50°C, add dropwise 9.2 grams of N,N-dimethylacetamide dimethyl acetamide under rapid stirring After the addition of aldehyde, keep warm at 40-50°C for 4 hours, remove part of the solvent under reduced pressure, cool down to below 20°C, add 9ml of water, adjust the pH of the reaction mixture to about 6.5 with acetic acid, and continue stirring for 30 minutes. Stop responding. Liquid chromatography detection showed that the content of sucrose-6-ethyl ester was 25.6%, and the yield was 77%.
Embodiment 3
[0026] Embodiment 3, the synthesis of sucrose-6-benzoate
[0027] Take 20 grams of sucrose, add it to a four-necked flask equipped with stirring, add 90ml of DMF, raise the temperature to 90°C, stir for 30 minutes, after the sucrose is completely dissolved, cool down to 40°C, and dropwise add 13 grams of N,N-dimethyl Benzyl benzamide dimethyl acetal, after the addition, keep warm at 40-50°C for 4.5 hours, remove part of the solvent under reduced pressure, cool down to below 20°C, add 9ml of water, adjust the pH value to about 6.5 with acetic acid, Stirring was continued for 30 minutes. Stop responding. As detected by liquid chromatography, the content of sucrose-6-benzoate was 30.5%, and the yield was 82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com