Polypeptide of controlling IIIv virus fusion and its use
A technology of virus envelope, -X1-X2WN-X3-X4-TWMEWER-X5-IE-X6-YTKLIY-X7-IL-X8-SQEX9-, applied in the field of polypeptides that inhibit virus fusion, can solve the problem of large molecular weight, Difficulties in synthesis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0110] Synthesis and purification of embodiment 1 polypeptide
[0111] The polypeptide provided by the present invention can be synthesized on an ABI 433 solid-phase synthesizer of Applied Systems Biosystems in the United States, and the modification of the polypeptide is done manually. The amino acid used in the synthesis is protected with Fmoc (product of Advanced Chemtech, USA), and the resin used is Rink resin (product of Advanced Chemtech, USA). During synthesis, 1-hydroxybenzotriazole (HoBt) (product of Advanced Chemtech, USA) was dissolved in N-methylpyrrolidone (NMP) (PE company) as an activator, and dicyclohexylcarbodiimide (DCC) was used (Acros Company) was used as a coupling agent, and piperidine (Piperidine) (Shanghai Jier Biochemical) was used to remove the protecting group. Amino acids all have an L-type chemical structure, and they are sequentially coupled to the Rink resin.
[0112]The usage amount of Rink resin and the usage amount of Fmc protected amino aci...
Embodiment 2
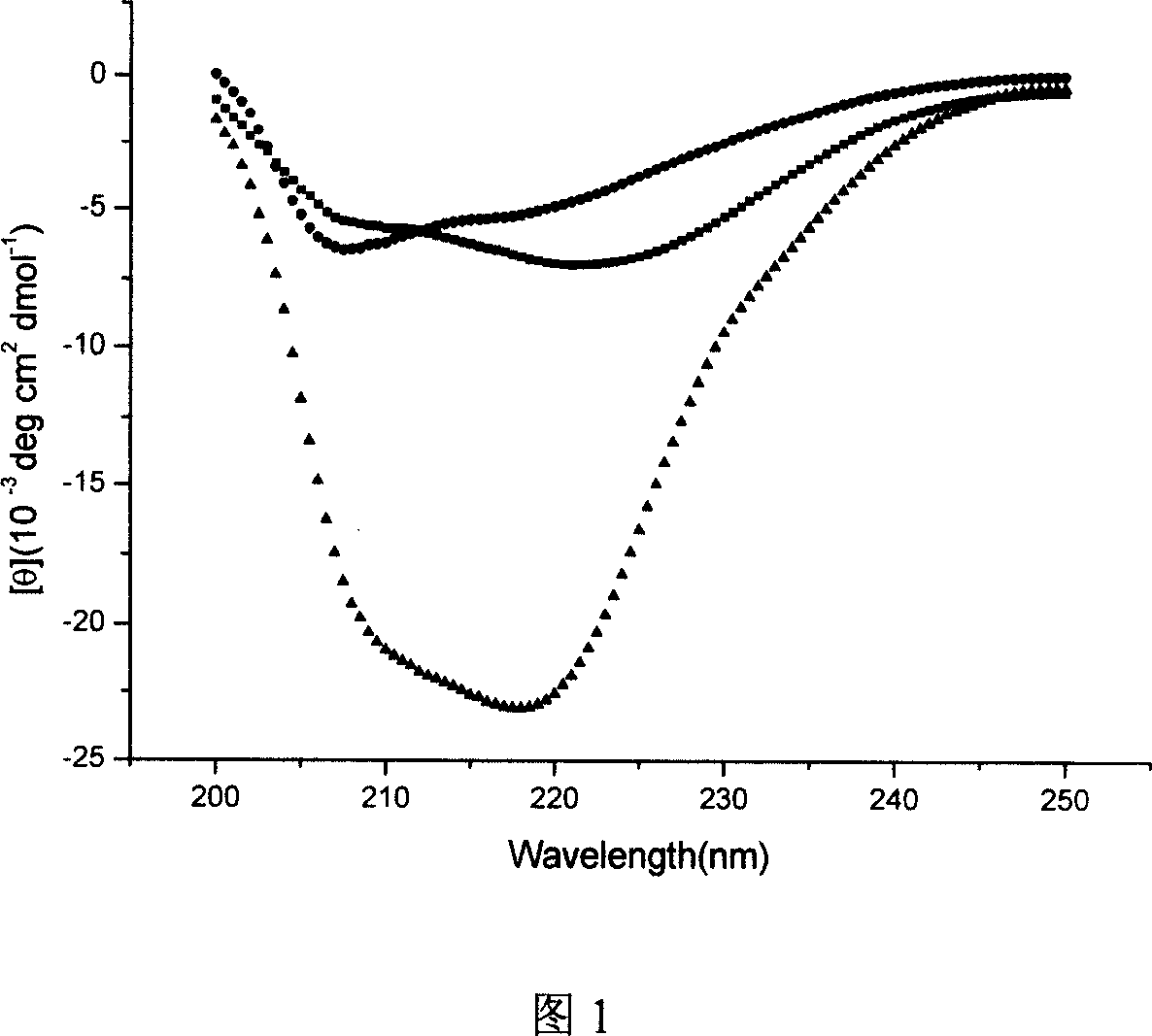
[0124] The CD spectrum determination of embodiment 2 polypeptide secondary structure
[0125] 1. Purpose of the experiment
[0126] By measuring the changes in the secondary structure (such as helical content) of the polypeptide provided by the invention and N36 (a sequence from the NHR region of HIV gp41, containing the action site of the polypeptide of the invention and T20, etc.) and their complexes To analyze the intermolecular interaction between the polypeptide of the present invention and N36. The stronger the interaction, the higher the activity, and vice versa.
[0127] 2. Experimental instruments, reagents and methods
[0128] Instrument: JASCO J-715-150L type
[0129] Parameter selection: resolution 0.1nm, wave width 5.0nm, response time 4.0s, wavelength 200-250nm
[0130] Dissolving buffer: 50mM NaH 2 PO 4 (Containing 150mM NaCl)pH=7.2
[0131] Peptide concentration: 10μM
[0132] The secondary structure determination of 18 polypeptides provided by the inve...
Embodiment 3
[0141] Example 3 Gel HPLC detection of the binding effect of the polypeptide of the present invention and N36
[0142] 1. Experimental purpose and principle
[0143] The purpose is to prove whether there is a strong interaction between the two by detecting whether the polypeptide of the present invention binds to N36.
[0144] In a gel column, substances are separated according to their molecular weights, and substances with larger molecular weights are eluted first. In this experiment, if the polypeptide forms a complex with N36, it will be eluted first after the molecular weight increases. If the complex cannot be formed, the mixture will form two peaks of equal height, corresponding to the polypeptide and N36 provided by the present invention respectively.
[0145] 2. Experimental instruments, reagents and methods
[0146] Gel column: TSK-G3000SWxl, 5μm, 7.8mm×300mm (product of Japan TOSOH company)
[0147] Instrument: Bio-Rad 13507 pump, Bio-Dimension TM UV / VIS detect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com