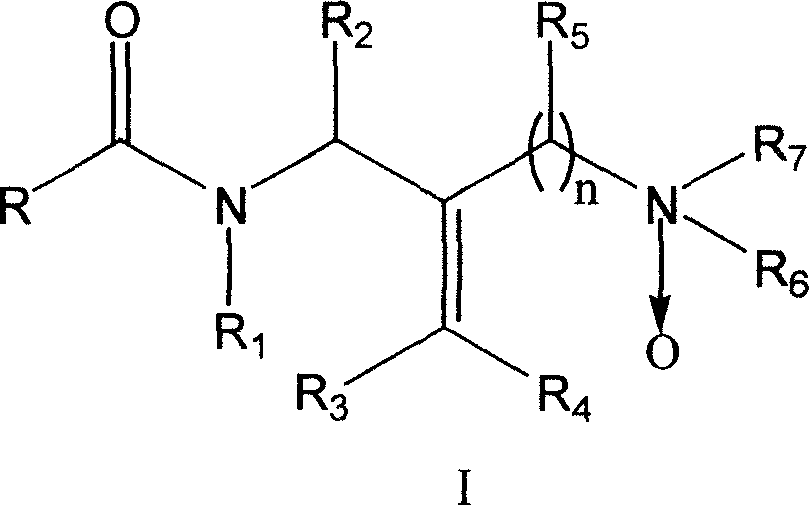
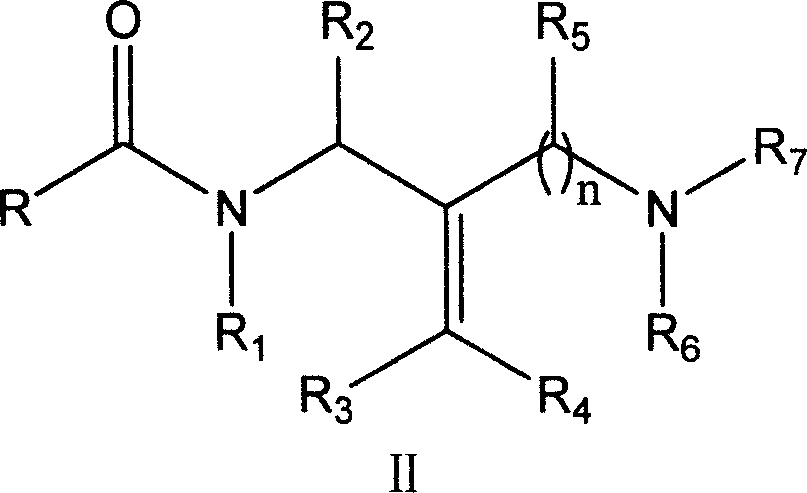
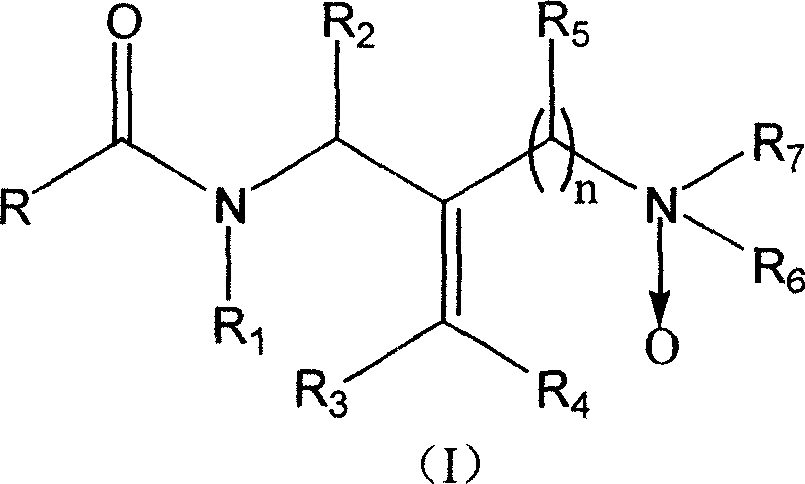
Diamine compound for resisting hepatitis B virus and its preparation method and its application
An amine compound and anti-hepatitis B technology, which is applied in the anti-hepatitis B virus diamine compound and its preparation and application field, can solve the problems of low glycyrrhizin content, complicated extraction and separation process, difficult quality control, etc., to achieve inhibition of hepatitis B virus, Simple synthetic route and low preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1: Preparation of (Z)-N-phenyl-N-(2-N', N'-dimethyloxyaminomethyl-3-phenylallyl)acetamide;
[0058] Take 20mL ethanol, weigh 0.500g (E)-N-phenyl-N-(2-N', N'-dimethylaminomethyl-3-phenylallyl) acetamide, add to 50mL round bottom In the flask, add 0.789g of 28% hydrogen peroxide to the above mixture, react at room temperature for 10 days, remove the solvent under reduced pressure, and vacuum dry to obtain (Z)-N-phenyl-N-(2-N', N'- Dimethyloxyaminomethyl-3-phenylallyl)acetamide 0.499g, yield 95%. IR (KBr, cm -1 ): 2952, 2861, 1662, 1494, 1389, 699; 1 HNMR (CDCl 3 , ppm.): 1.88 (s, 3H), 2.53 (s, 6H), 4.28 (d, 2H), 5.12 (d, 2H), 7.02-7.43 (m, 10H), 8.02 (s, 1H).
Embodiment 2
[0059] Example 2: (Z)-N-(2-methoxyphenyl)-N-(2-N', N'-dimethyloxyaminomethyl-3-phenylallyl)acetamide preparation of
[0060] Take 20mL ethanol, weigh 0.500g (E)-N-(2-methoxyphenyl)-N-(2-N', N'-dimethylaminomethyl-3-phenylallyl) ethyl Add amide into a 50mL round bottom flask, add 0.718g28% hydrogen peroxide to the above mixture, react at room temperature for 10 days, remove the solvent under reduced pressure, and vacuum dry to obtain (Z)-N-(2-methoxybenzene Base)-N-(2-N',N'-dimethyloxyaminomethyl-3-phenylallyl)acetamide 0.492g, yield 94%. IR (KBr, cm -1 ): 2953, 2847, 1662, 1499, 1389, 752; 1 HNMR (CDCl 3 , ppm.): 1.84(s, 3H), 2.51(s, 6H), 3.715(s, 3H), 4.21-4.32(m, 2H), 4.99-5.13(m, 2H), 7.00-7.38(m, 9H), 7.91 (s, 1H).
Embodiment 3
[0061] Example 3: Preparation of (Z)-N-(4-fluorophenyl)-N-(2-N', N'-dimethyloxyaminomethyl-3-phenylallyl)acetamide ;
[0062] Take 20mL of ethanol, weigh 0.500g of (E)-N-(4-fluorophenyl)-N-(2-N',N'-dimethylaminomethyl-3-phenylallyl)acetamide, Add it into a 50mL round bottom flask, add 0.745g of 28% hydrogen peroxide to the above mixture, react at room temperature for 10 days, remove the solvent under reduced pressure, and vacuum dry to obtain (Z)-N-(4-fluorophenyl)-N -(2-N', N'-dimethyloxyaminomethyl-3-phenylallyl)acetamide 0.477g, yield 91%. IR (KBr, cm -1 ): 2956, 2863, 1639, 1508, 1383, 704; 1 HNMR (CDCl 3 , ppm.): 1.85(s, 3H), 2.54(s, 6H), 4.21-4.32(m, 2H), 4.97-5.17(m, 2H), 6.70(s, 1H), 7.02-7.43(m, 9H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com