Lipid microsphere, lyophilized lipid microsphere containing docetaxel and preparation process thereof
A technology of docetaxel and lipid microspheres, which is applied in the directions of non-active ingredient medical preparations, liposome delivery, pharmaceutical formulations, etc., can solve the problems of inconvenient medication, low solubility, and poor patient compliance. Facilitate clinical application and improve solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
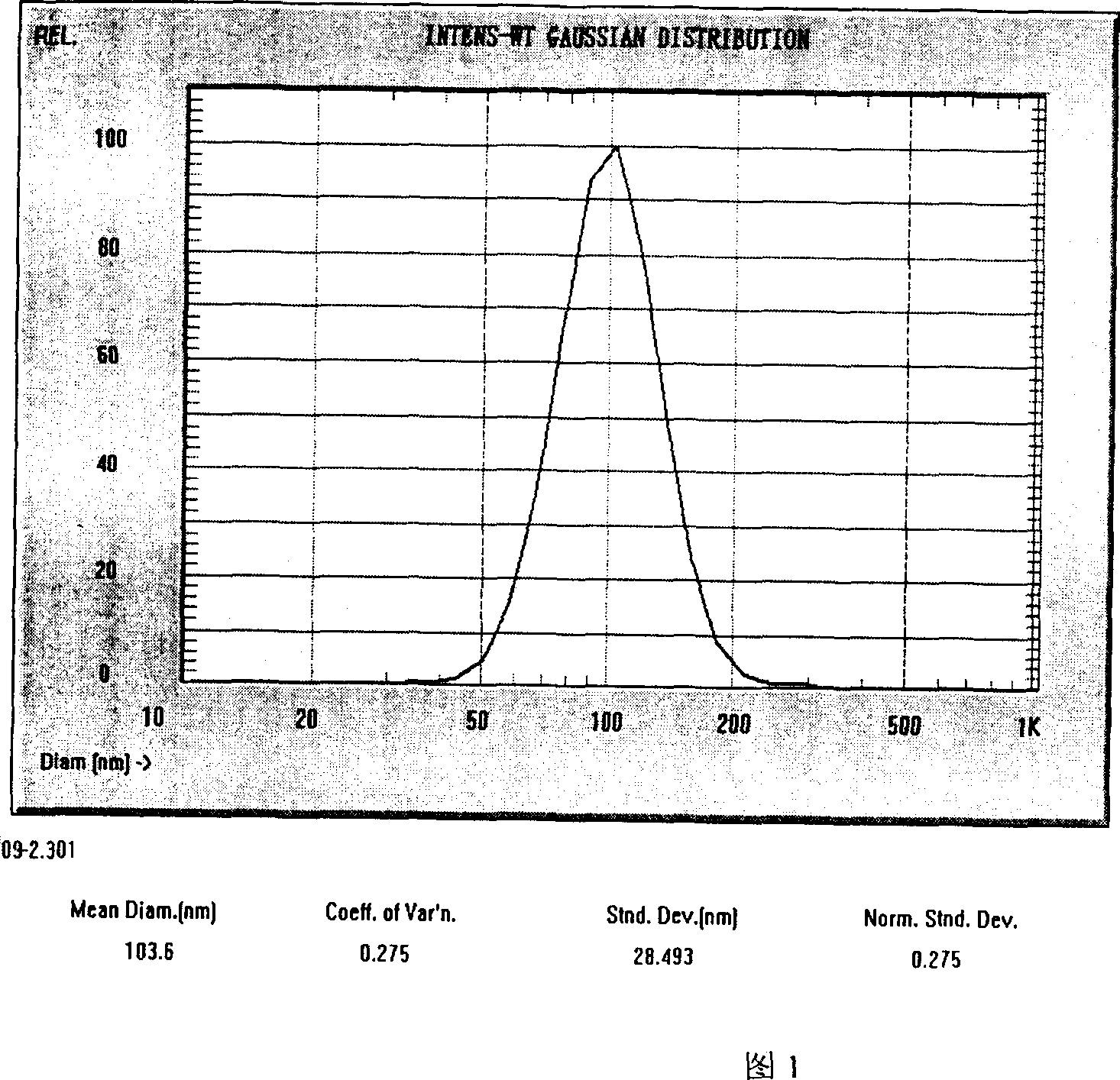
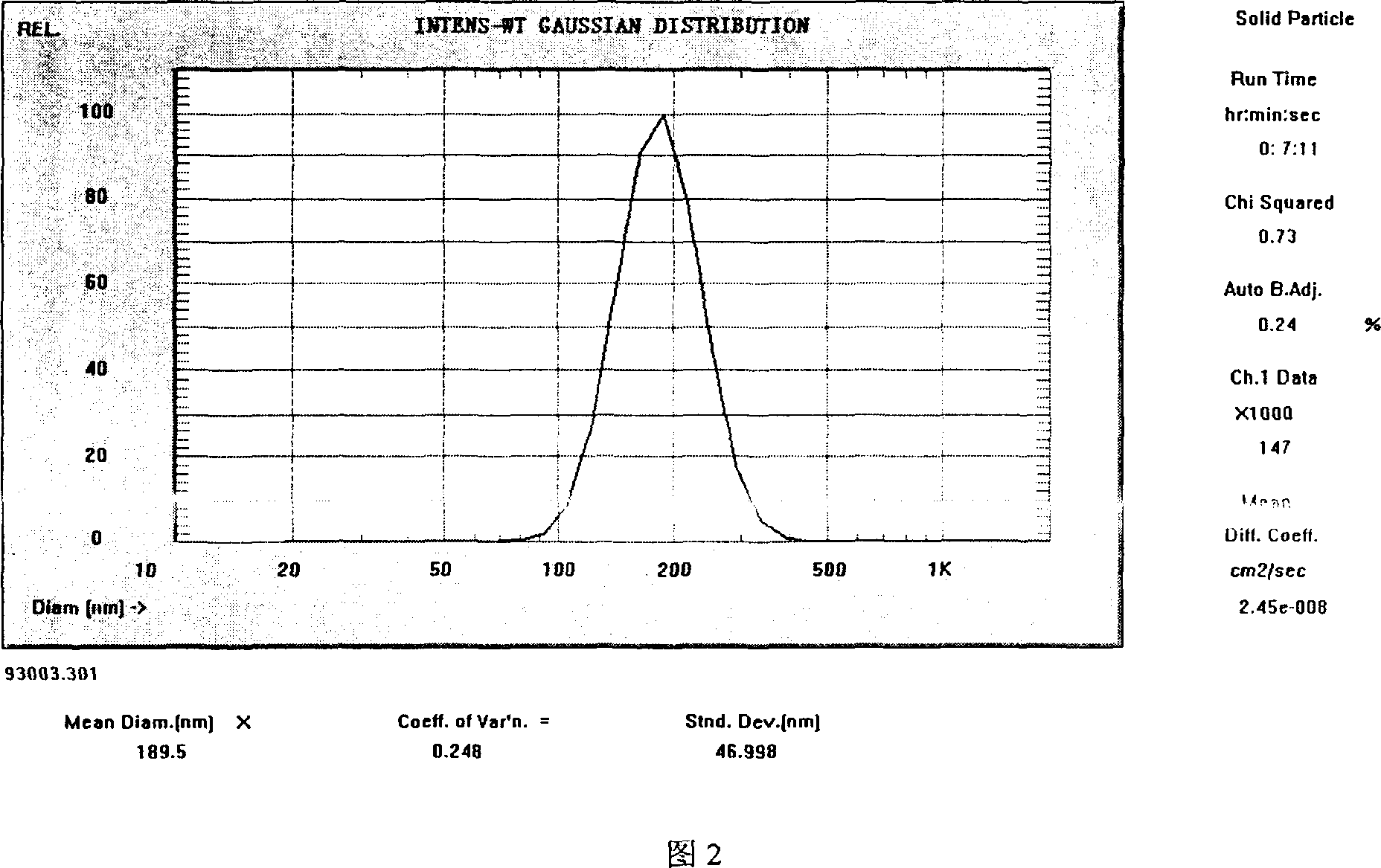
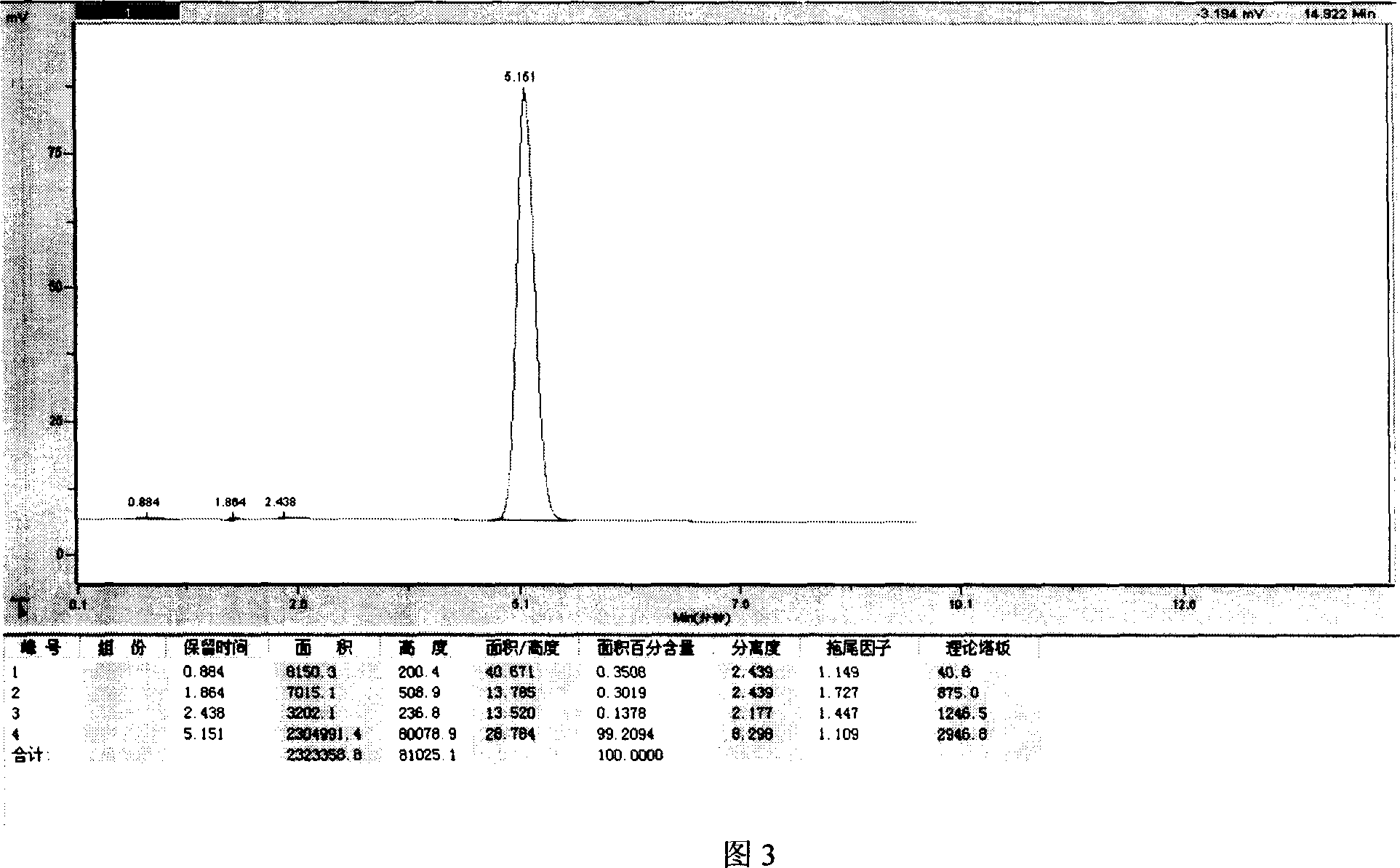
Image
Examples
Embodiment 1
[0084] Prescription composition:
[0085] Docetaxel 10g
[0086] VE 100g
[0087] Phospholipids 25g
[0088] Oleic acid 50g
[0089] PEG400 25g
[0090] Sucrose 50g
[0091] Disodium EDTA 0.05g
[0092]Add water for injection to 1000ml
[0093] Precisely weigh the raw and auxiliary materials; under the condition of nitrogen protection, add docetaxel, PEG400, oleic acid, VE, phospholipids, into the oily ingredient preparation tank, stir until they are all dissolved, and keep the solution temperature at 50°C to obtain Oil phase; add the prescribed amount of sucrose and disodium EDTA, and a certain amount of water for injection into the water phase preparation tank, stir and dissolve to keep the solution temperature at 50°C to obtain the water phase; add the water phase solution to the high-speed shear In the cutting equipment, slowly add the oil phase solution to the stirring water phase, keep the temperature at 45°C, stir and shear for 15 minu...
Embodiment 2
[0147] Prescription composition:
[0148] Docetaxel 10g
[0149] VE 100g
[0150] Phospholipids 25g
[0151] Oleic acid 50g
[0152] PEG400 25g
[0153] Sucrose 50g
[0154] Disodium EDTA 0.05g
[0155] Mannitol 100g
[0156] Glycine 20g
[0157] Add water for injection to 1000ml
[0158] Precisely weigh the raw and auxiliary materials; under the condition of nitrogen protection, add docetaxel, phospholipids, PEG400, oleic acid, and VE into the oily ingredient preparation tank, stir until completely dissolved, and keep the solution temperature at 50°C to obtain oil phase; add the prescribed amount of sucrose and disodium EDTA, and a certain amount of water for injection into the water phase preparation tank, stir, dissolve and keep the solution temperature at 50°C to obtain the water phase; add the water phase solution to the high-speed shear In the equipment, slowly add the oil phase solution to the stirring water phase, keep the...
Embodiment 3
[0161] Prescription composition:
[0162] Docetaxel 2g
[0163] VE 80g
[0164] Phospholipids 20g
[0165] Polyoxyethylene castor oil 30g
[0166] Add water for injection to 1000ml
[0167] The preparation method was the same as in Example 1 to prepare docetaxel lipid microspheres.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com