Compound preparation for enhancing memory
A technology for enhancing memory and compound preparations, which can be applied to medical preparations containing active ingredients, pharmaceutical formulations, medical raw materials derived from Ginkgo biloba, etc., and can solve the problems of permanent damage and elevation of central nervous cells.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] Embodiment one sample formula
[0113] This embodiment provides samples 1-6 for comparative tests, wherein sample 1 includes all the necessary active ingredients screened out, sample 2 does not include the active ingredient for controlling oxidative stress, i.e. adjuvant, and sample 3 is Including various ingredients of monarch and minister adjuvant drugs, but the compound preparation containing the least active ingredients obtained through optimization, sample 4 does not include the active ingredient for enhancing the oxygen supply of the central nervous system of the brain, that is, the main minister drug part, sample 5 does not Including the primary drug, Sample 6 is only an adjuvant that is an active ingredient for controlling oxidative stress.
[0114] The active ingredients and contents involved in the samples are shown in Table 1:
[0115] Units not indicated in Table 1 are milligrams
[0116] Table I
[0117]
[0118] L-tryptophan
Embodiment 2
[0119] Embodiment two efficacy application test
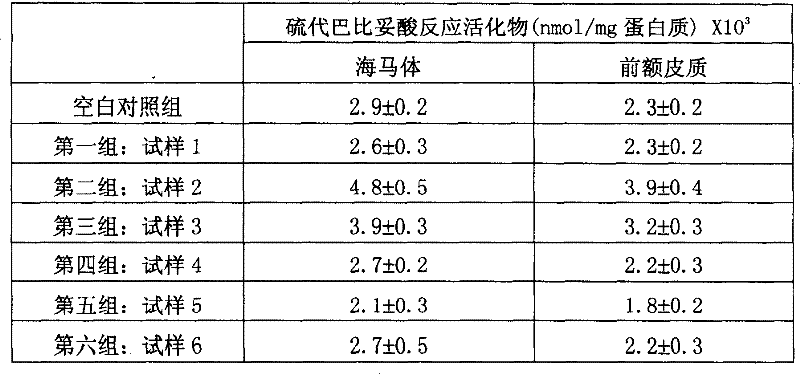
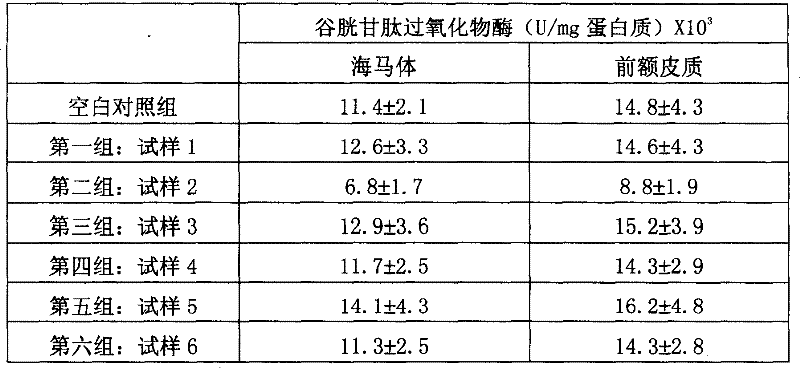
[0120] Comparison of Oxidative Stress States in the Brain
[0121] Animal experiments were carried out with the sample composition of Example 1 to test the effect of these samples on oxidative stress. Animals Six-month-old WISTAR male mice were used. Test rats were housed in a standard animal testing environment, with water and food readily available, on a 12-hour day / night cycle. They were divided into seven groups with 10 rats in each group. The first group is the blank group, which is fed with saline; the test mice in the other groups are fed with the preparations of samples 1-6. The feeding amount was compared with 5-hydroxytryptophan or acetyl-L-carnitine in the sample formulation (1.5 mg / kg body weight). Eight hours later, the test mice and the control group were all killed by head and neck dislocation under ether anesthesia. The prefrontal cortex and hippocampus in the rat brain were picked under ice-cold conditions...
Embodiment 3
[0134] Effects on learning ability and memory (normal state of health, and abnormal state of brain cognitive deficits)
[0135] A: Object Recognition Test in Normal Health
[0136] The test used 10-week-old C57BL / 6J male mice, and the test mice were randomly divided into eight groups, ten in each group. The first group is a blank group, fed with saline; the second group is a positive control group, intraperitoneally injected Rolipram (Rolipram, 10% DMSO solution, 0.1mg / kg body weight); all the other groups of test mice are fed with sample 1-6 preparations . The feeding amount was compared with 5-hydroxytryptophan or acetyl-L-carnitine in the sample formulation (1.5 mg / kg body weight). 60 minutes before the test if fed or 20 minutes before the test if injected.
[0137] All test rats were put into the test environment for at least one hour before the test to familiarize themselves with the environment. At this time, there were two small external objects in the test environme...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com