Compound cantharis drop pills and its preparation method
A Cantharidin Dropping Pill and Cantharidin Technology, which is applied in the directions of pill delivery, pharmaceutical formulations, and medical preparations containing active ingredients, etc., can solve problems such as difficulty in adapting to patients with difficulty in swallowing, affecting the full play of drug efficacy, and low bioavailability. , to achieve the benefits of labor protection and environmental protection, improve bioavailability, and good drug stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment one: except bear bile powder 0.96g, ginseng 23.8g, dogwood 47.6g, Ligustrum lucidum 47.6g, Scutellaria barbata 142.8g, add 60~80% ethanol, the first time is 8 times the amount, the second time The amount is 5 times that of the third time, heated to reflux 3 times, each time for 2 hours, filtered, and the filtrate recovered ethanol and concentrated to extract. 9.52g of mylabris was soaked and extracted 3 times with chloroform, each dosage was 38ml, soaked for 72 hours, the extracts were combined, the chloroform was recovered, and concentrated to extract. Take 119g of astragalus, 119g of Acanthopanax, 38g of Sanleng, 38g of zedoary, and 23.8g of licorice, add water to decoct three times, the amount of water added is 8 times the amount for the first time, and the amount for the second and third times is 5 times each. 3 hours for the first time, 1.5 hours for the second time, and 1 hour for the third time, combine the decoction, filter, and concentrate the filtra...
Embodiment 2
[0021] Example 2: In addition to 0.96g of bear bile powder, 23.8g of ginseng, 47.6g of dogwood, 47.6g of Ligustrum lucidum, 142.8g of Scutellaria barbata, 119g of astragalus, 119g of Acanthopanax, 38g of Sanleng, 38g of curcuma, and 23.8g of licorice g, add 65% ethanol, add 8 times the amount for the first time, add 6 times the amount for the second time, add 4 times the amount for the third time, heat and reflux 3 times, each time for 2 hours, filter, and the filtrate recovers ethanol and concentrates to extract. 9.52g of mylabris was soaked and extracted 3 times with chloroform, each dosage was 38ml, soaked for 72 hours, the extracts were combined, the chloroform was recovered, and concentrated to extract. After dissolving 0.96 g of bear bile powder in 10 ml of 80° C. water, add it to the extract, mix evenly, and vacuum-dry at a temperature of 75° C. to obtain the compound mylabris medicinal powder. Get compound mylabris medicinal powder, pulverize, cross 120 mesh sieves. ...
Embodiment 3
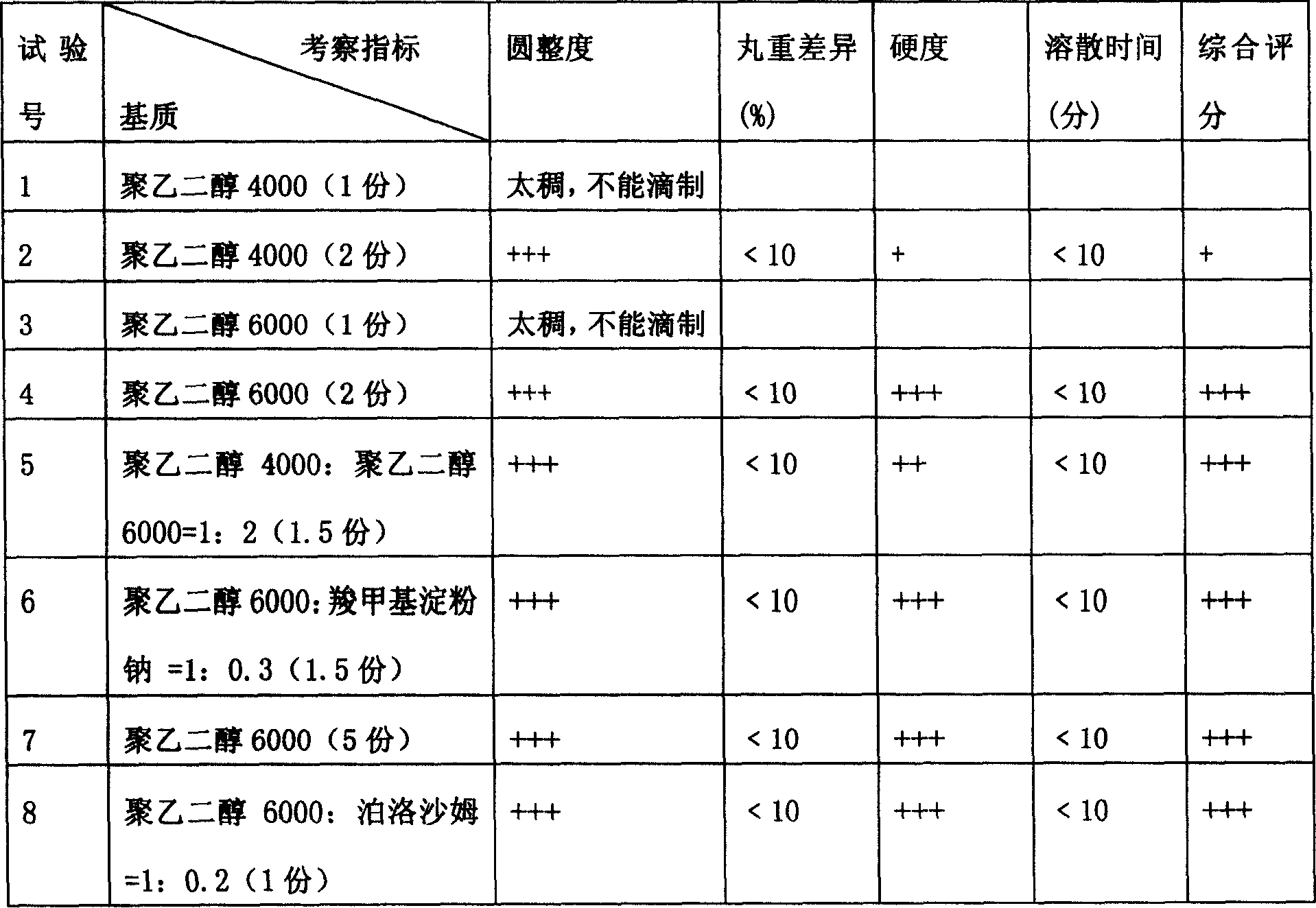
[0022] Embodiment three: the present embodiment investigates indexes such as roundness, pill weight difference, hardness, dissolution time by compound recipe mylabris medicinal powder and different substrate formulas, to determine the weight ratio of compound recipe myracari medicinal powder and single or mixed substrate.
[0023] Compound myracari powder and different matrix formula test (compound mylabris powder each 1 part)
[0024]
[0025] Note: 1. The cooling agent is simethicone oil or liquid paraffin, and the cooling temperature is 0-5°C; the heat preservation temperature of the drug material and the dripper is 75-80°C; the dripping speed is 30-50 capsules / min.
[0026]2. The above results show that the indicators of No. 4, 5, 6, 7, and 8 tests are all good, that is, the compound mylabris powder and the matrix ratio can be smoothly dripped when the ratio is 1:1-1:5. However, considering factors such as dosage, the preferred ratio is No. 4, 5, 6, and No. 8 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com