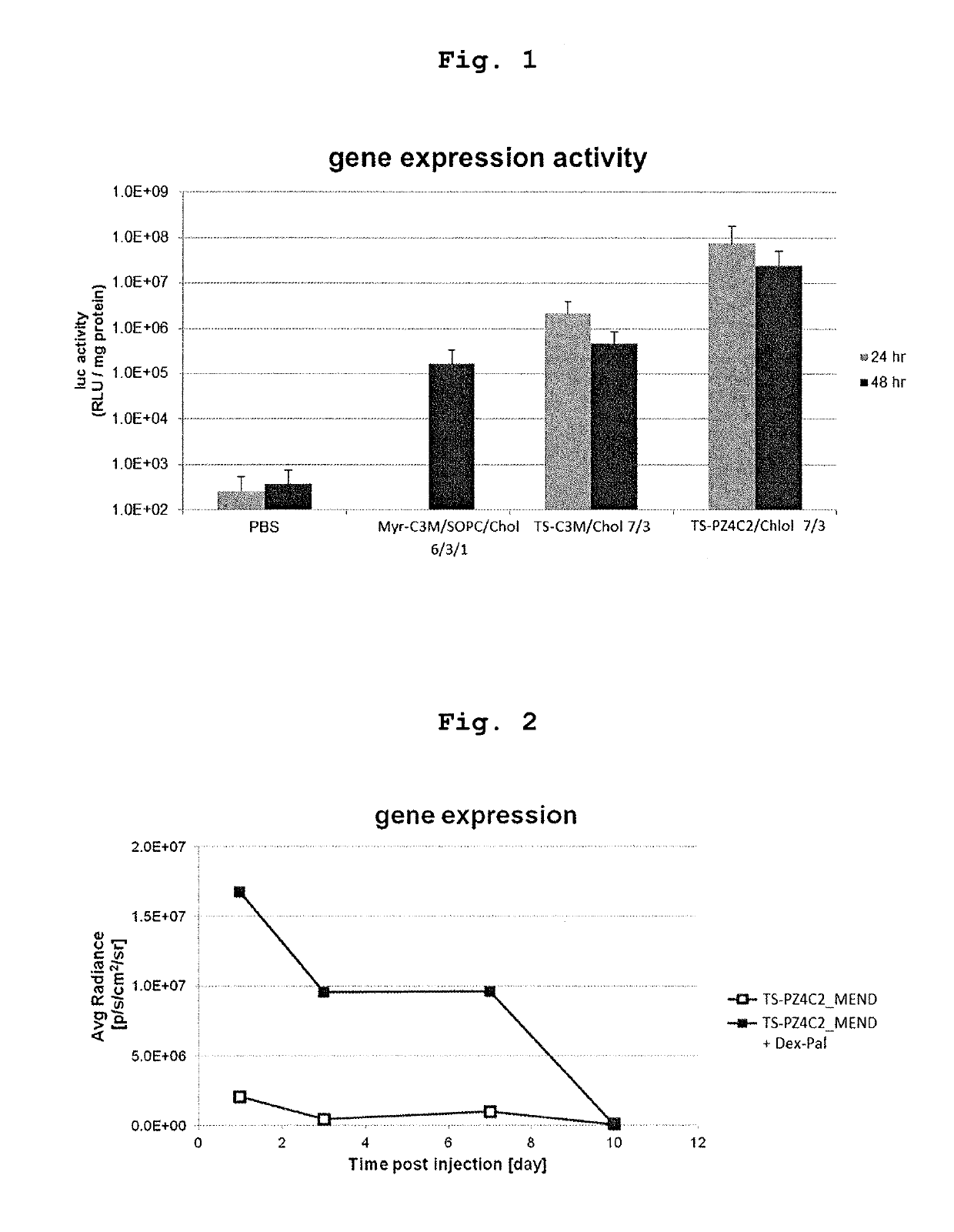
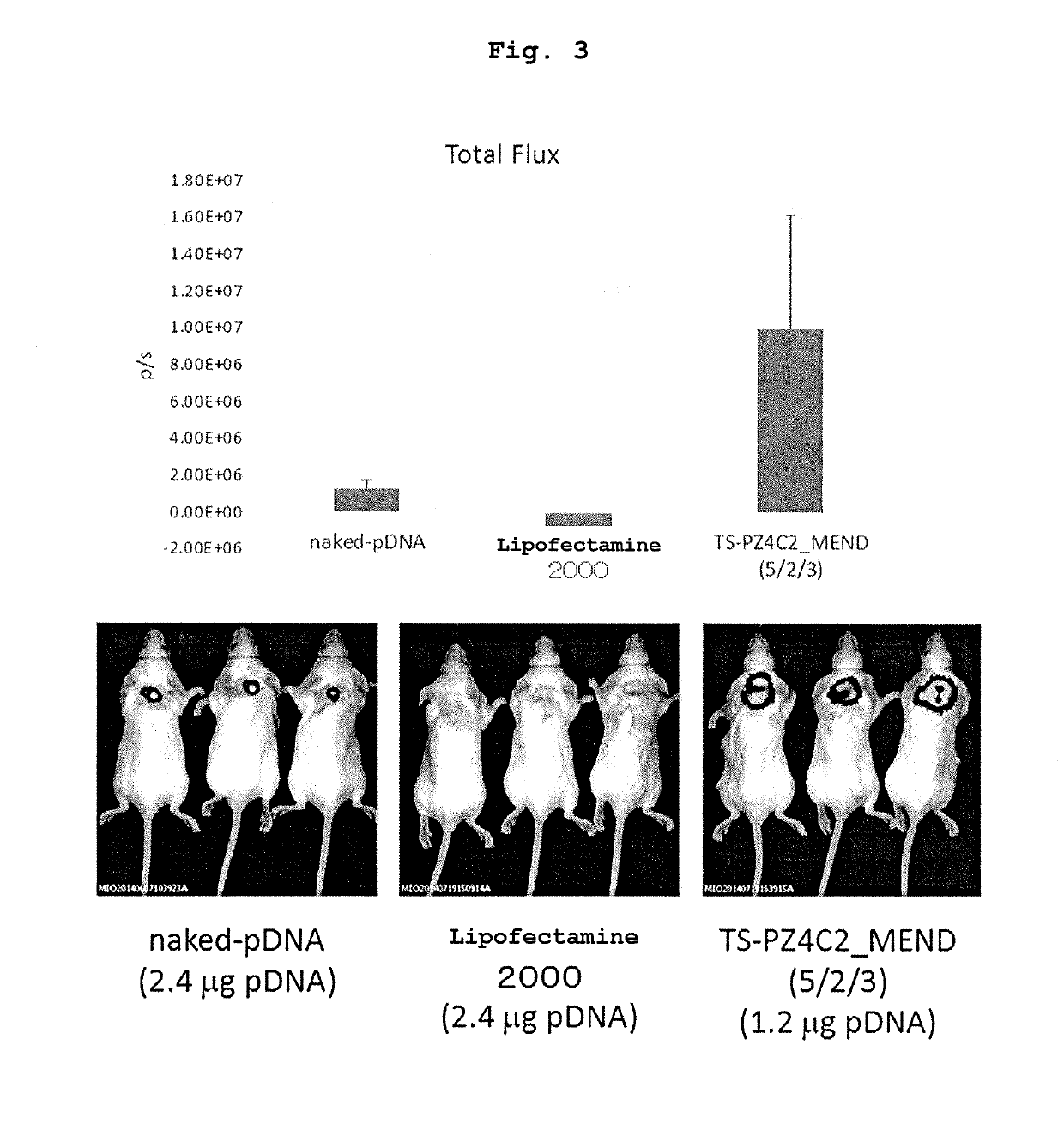
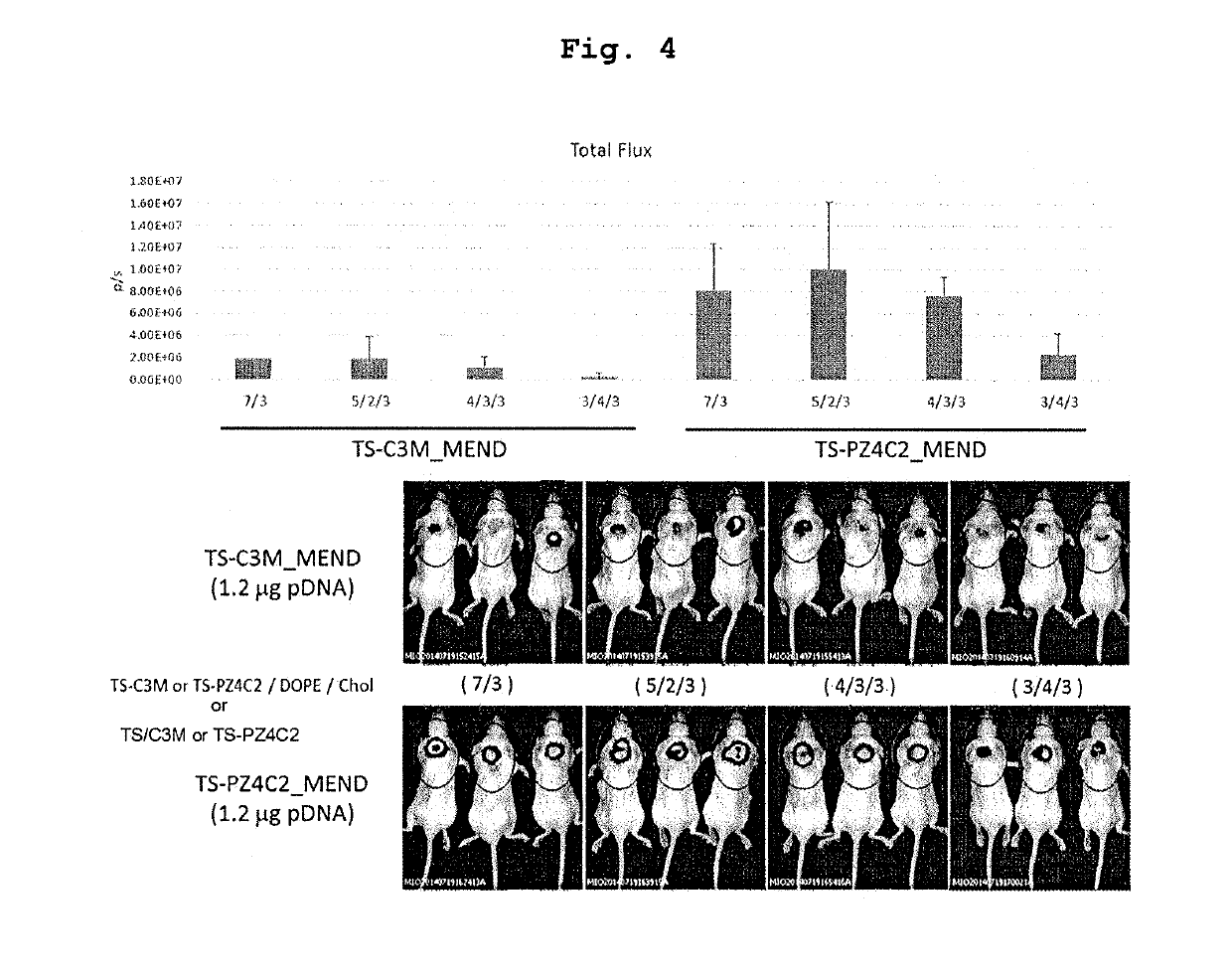
Cationic lipid
a lipid and cationic technology, applied in the field of cationic lipids, can solve the problems of affecting the nucleic acid delivery efficiency of the cytoplasm, the difficulty of nucleic acid delivery into cells, and the achievement of nucleic acid delivery efficiency into the cytoplasm, so as to promote the release of the encapsulated substance (nucleic acid), suppress the degradation of nucleic acid by serum components, and promote the effect of encapsulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[Example 1] Synthesis of TS-PZ4C2
[0176]Acetonitrile (143 ml) was added to bis(2-hydroxyethyl) disulfide (15 g, manufactured by Tokyo Chemical Industry Co., Ltd.) (97 mmol), and the mixture was dissolved at 20-25° C. Triethylamine (33.3 g, manufactured by KANTO CHEMICAL CO., INC.) (328 mmol) was added, and the mixture was cooled to 10° C. with stirring. Methanesulfonyl chloride (34.5 g, manufactured by KANTO CHEMICAL CO., INC.) (300 mmol) was added dropwise over 1 hr to set the temperature to 20° C. or below. After the completion of the dropwise addition, the mixture was reacted at 20-25° C. for 3 hr. The disappearance of the spot of bis(2-hydroxyethyl) disulfide was confirmed by TLC analysis (eluent: chloroform, iodine color development), and the reaction was completed. Ethanol (29 mL) was added to the reaction solution to discontinue the reaction, and insoluble materials were removed by filtration. 10% Sodium bicarbonate water (150 g) was added to the filtrate, and the mixture was ...
example 2
[Example 2] Synthesis of L-PZ4C2
[0185]di-PZ4C2 form (2.5 g, 7 mmol) and linoleic acid (3.7 g, manufactured by NOF CORPORATION) (13 mmol) were dissolved in chloroform (25 mL) at 20-25° C. Thereafter, 4-dimethylaminopyridine (0.3 g, 3 mmol) and EDC (3.8 g, 20 mmol) were added and the mixture was reacted at 30° C. for 4 hr. The disappearance of the spot of linoleic acid was confirmed by TLC analysis (eluent: chloroform / methanol=9 / 1 (v / v), phosphoric acid copper sulfate color development), and the reaction was completed. The reaction solvent was distilled off by an evaporator, and hexane (57 mL) was added. Thereafter, acetonitrile (24 mL) was added, and the mixture was stirred for 5 min. After standing for 10 min, the hexane layer was recovered, and the solvent was distilled off by an evaporator to give a pale-yellow liquid (4.9 g). The liquid (4.9 g) was purified by silica gel column chromatography (eluent: chloroform / methanol=99 / 1-97 / 3 (v / v)) to give the object product L-PZ4C2 (3.1 g)...
example 3
[Example 3] Synthesis of O-PZ4C2
[0188]di-PZ4C2 form (0.8 g, 2 mmol) and oleic acid (1.2 g, manufactured by NOF CORPORATION) (4 mmol) were dissolved in chloroform (8 mL) at 20-25° C. Thereafter, 4-dimethylaminopyridine (0.1 g, 1 mmol) and EDC (1.2 g, 6 mmol) were added and the mixture was reacted at 30° C. for 3 hr. The disappearance of the spot of oleic acid was confirmed by TLC analysis (eluent: chloroform / methanol=9 / 1 (v / v), phosphoric acid copper sulfate color development), and the reaction was completed. The reaction solvent was distilled off by an evaporator, and hexane (12 mL) was added. Thereafter, acetonitrile (5 mL) was added, and the mixture was stirred for 5 min. After standing for 10 min, the hexane layer was recovered, and the solvent was distilled off by an evaporator to give a pale-yellow liquid (1.8 g). The liquid (1.7 g) was purified by silica gel column chromatography (eluent: chloroform / methanol=99 / 1-97 / 3 (v / v)) to give the object product, O-PZ4C2 (1.1 g).
1H-NMR S...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com