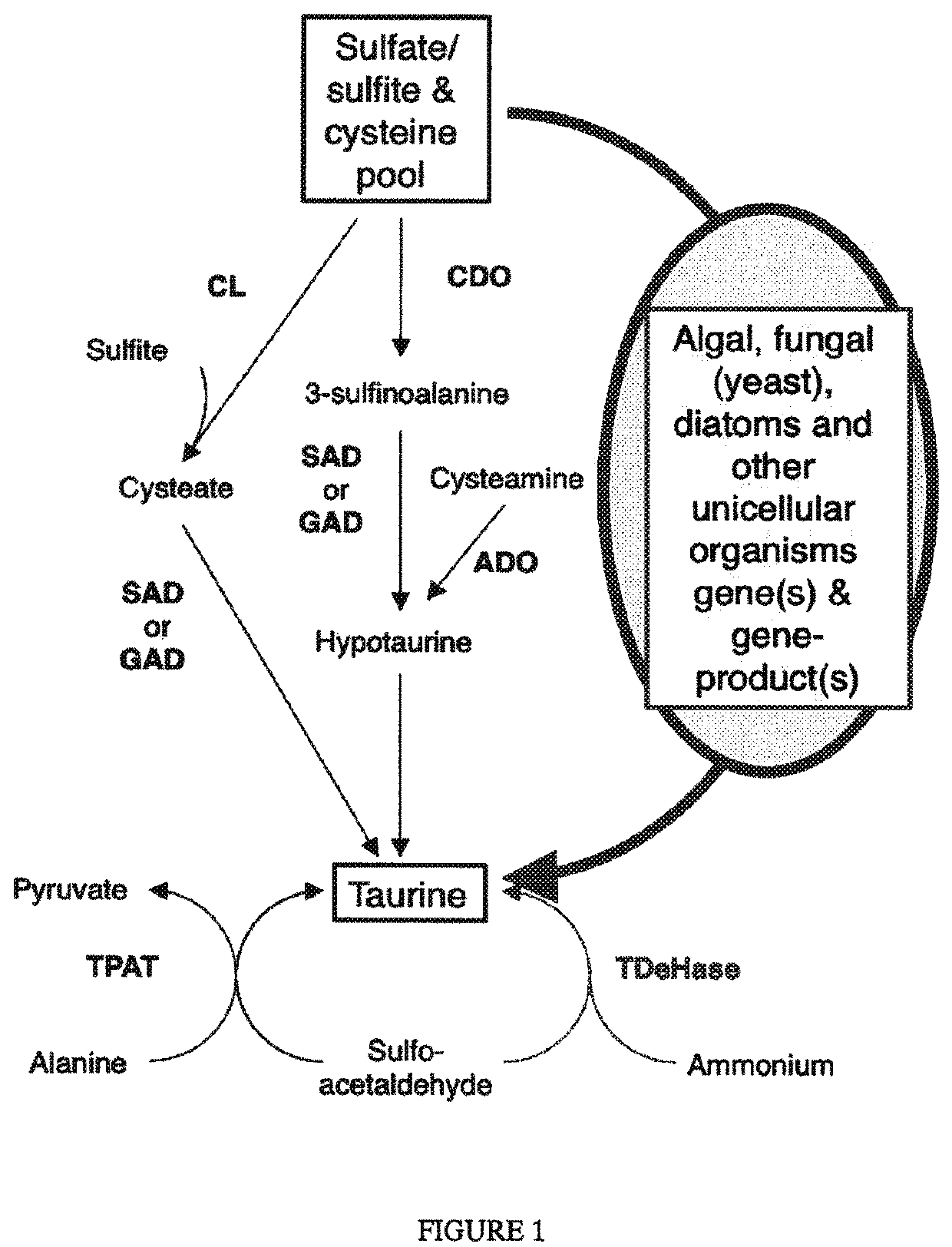
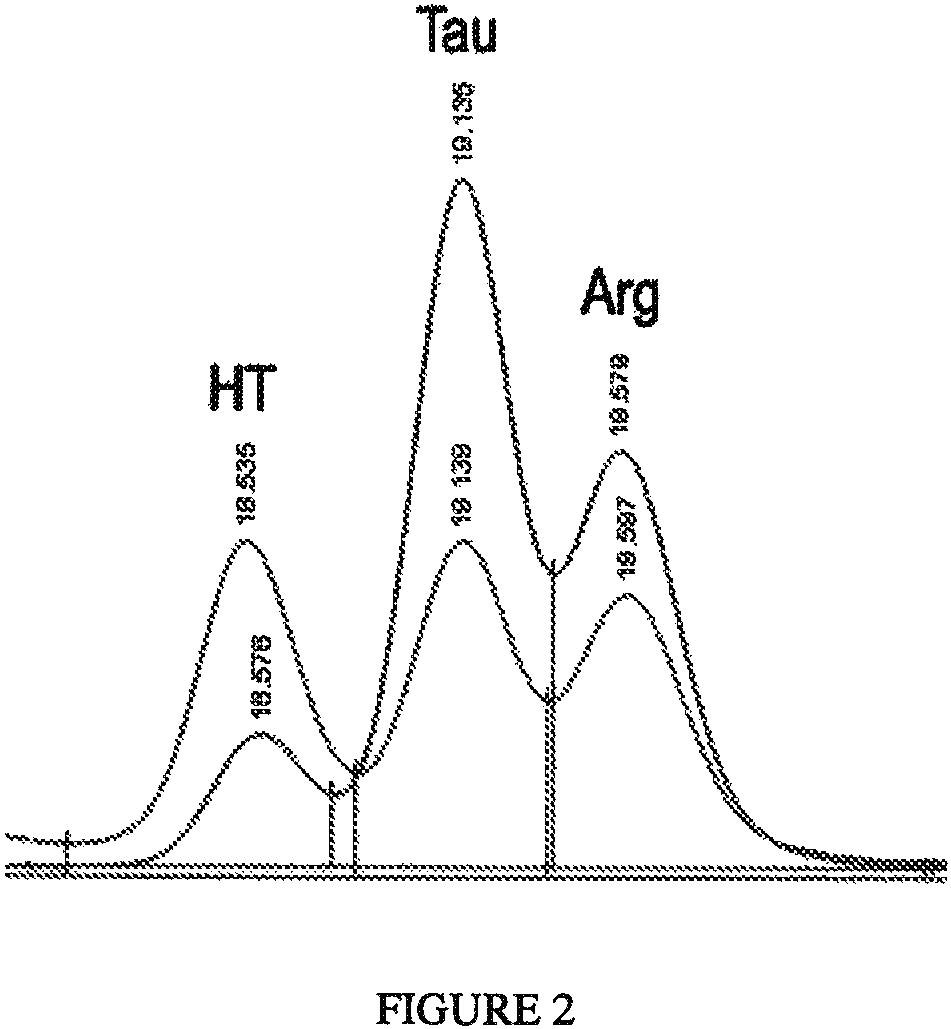
Algal and fungal genes and their uses for taurine biosynthesis in cells
a technology of taurine and genes, applied in the field of recombinant production of taurine, can solve the problems of never providing validation, never divulging the sequences of genes or the sequences of corresponding peptides, etc., and achieves the effects of improving the characteristics of food, increasing the level of sulfur-containing compounds, and increasing nutritional valu
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Development of a Transgenic Plant that Constitutively Expresses CDOL with the Native Transit Peptide and Constitutively Expresses SADL with the Native Transit Peptide in Tandem with Independent Promoters
[0471]Step 1: Use chemical synthesis to make a DNA construct that contains a constitutive promoter, 35S, fused with the nucleotide sequence for CDOL gene (SEQ ID NO:1, SEQ ID NO:2, SEQ ID NO:3, or SEQ ID NO:4) and a NOS terminator. Clone the DNA construct into a binary vector, such as pCambia1300, pCambia2300, or pCambia3200.
[0472]The CDOL gene is as follows:[0473]a. Derived from SEQ ID NO:1, optimized for expression in Arabidopsis or soybean (dicots) or corn (a monocot), and encoding a CDOL peptide from Chlamydomonas reinhardtii (SEQ ID NO:5); or[0474]b. Derived from SEQ ID NO:2, optimized for expression in Arabidopsis or soybean (dicots) or corn (a monocot), and encoding a CDOL peptide from Guillardia theta (SEQ ID NO:6); or[0475]c. Derived from SEQ ID NO:3, optimized for expressio...
example 2
Development of a Transgenic Plant that Constitutively Expresses CDOL with a Plant Plastid Transit Peptide and Constitutively Expresses SADL Protein with a Plant Plastid Transit Peptide in Tandem with Independent Promoters
[0485]Step 1: Use chemical synthesis to make a DNA construct that contains a constitutive promoter, 35S, fused with the nucleotide sequence for a plastid transit peptide (SEQ ID NO:21), truncated CDOL gene (SEQ ID NO:1, SEQ ID NO:2, SEQ ID NO:3, or SEQ ID NO:4) and a NOS terminator. Clone the DNA construct into a binary vector, such as pCambia1300, pCambia2300, or pCambia3200.
[0486]The nucleotide sequence for the plastid transit peptide (SEQ ID NO:21) encodes the peptide SEQ ID NO:22.
[0487]The CDOL gene is as follows:[0488]a. Derived from SEQ ID NO:1 by removing nucleotides 1 through 159 (corresponding to the native transit peptide), optimized for expression in Arabidopsis or soybean (dicots) or corn (a monocot), and encoding a CDOL peptide from Chlamydomonas reinha...
example 3
Development of a Transgenic Plant that Constitutively Expresses CDOL with the Native Plastid Transit Peptide and Constitutively Expresses CS / PLP-DC with the Native Transit Peptide in Tandem with Independent Promotes
[0501]Step 1: Use chemical synthesis to make a DNA construct that contains a constitutive promoter, 35S, fused with the nucleotide sequence for CDOL gene (SEQ ID NO:1, SEQ ID NO:2, SEQ ID NO:3, or SEQ ID NO:4) and a NOS terminator. Clone the DNA construct into a binary vector, such as pCambia1300, pCambia2300, or pCambia3200.
[0502]The CDOL gene is as follows:[0503]a. Derived from SEQ ID NO:1, optimized for expression in Arabidopsis or soybean (dicots) or corn (a monocot), and encoding a CDOL peptide from Chlamydomonas reinhardtii (SEQ ID NO:5); or[0504]b. Derived from SEQ ID NO:2, optimized for expression in Arabidopsis or soybean (dicots) or corn (a monocot), and encoding a CDOL peptide from Guillardia theta (SEQ ID NO:6); or[0505]c. Derived from SEQ ID NO:3, optimized f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com