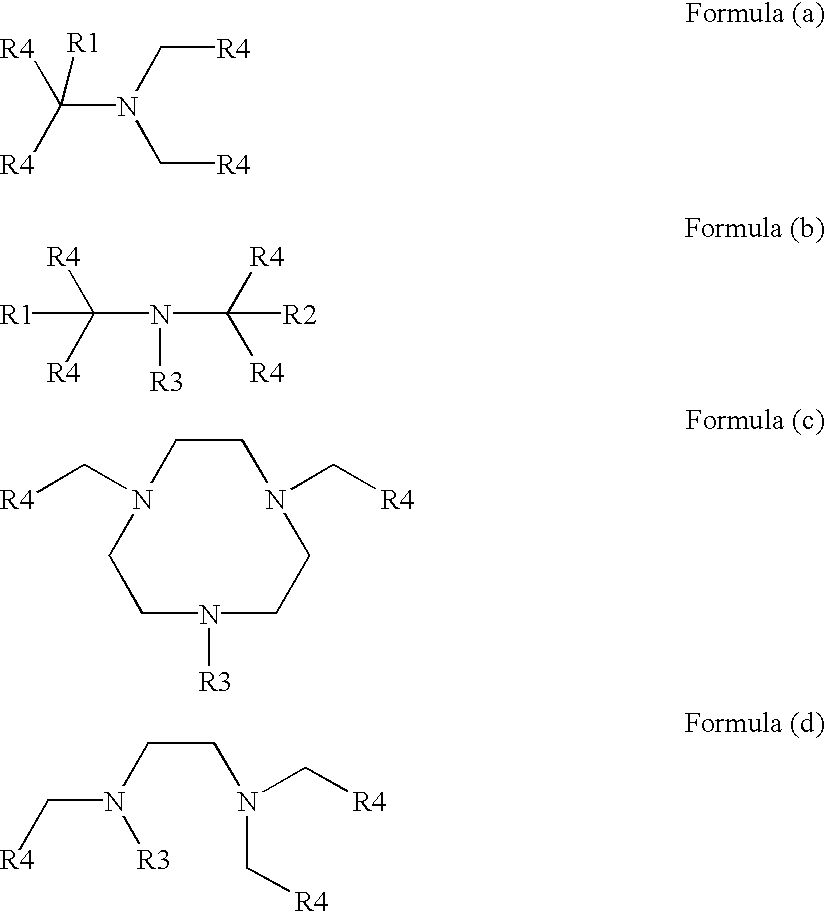
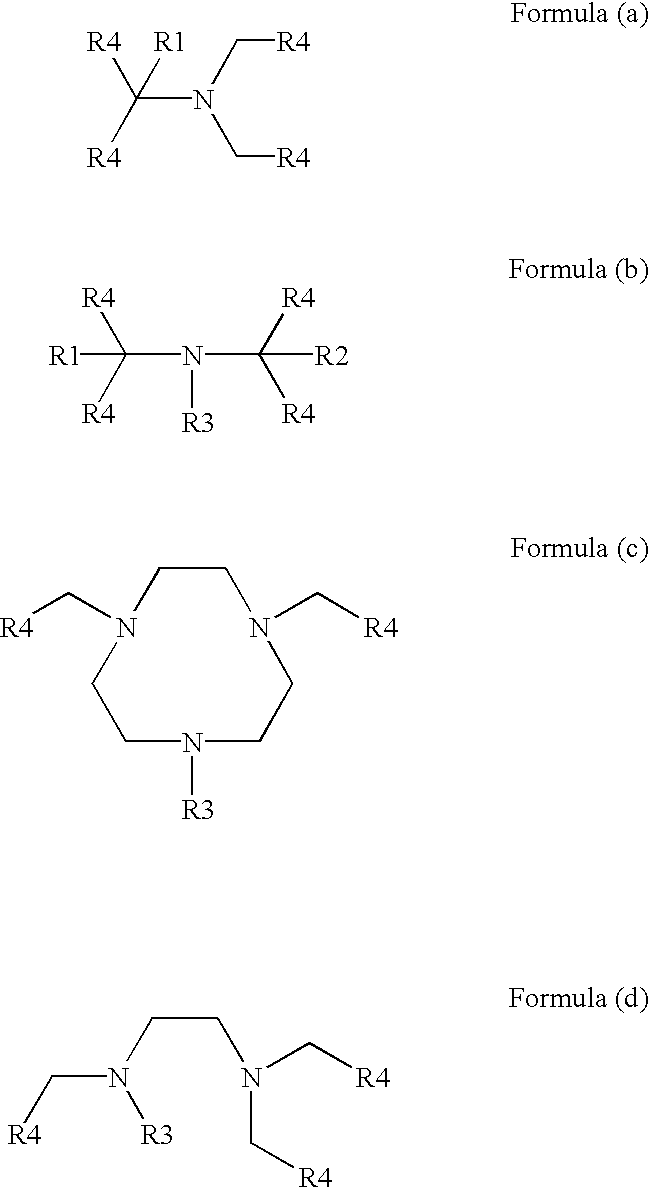
Transition metal complexes with polydentate ligands for enhancing the bleaching and delignifying effect of peroxo compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0097] Synthesis of {[Co(N4Py)].sub.2O.sub.2}Cl.sub.2(ClO.sub.4).sub.2, referred to below as (Co--N4Py).sub.2(.mu.-O.sub.2)
[0098] A solution of 1.43 g (3.89 mmol) of the ligand N4Py in a small amount of methanol (approx. 5 ml) was added at room temperature to a solution of 925 milligrams (mg) (3.89 mmol) of CoCl.sub.2.times.6H.sub.2O and 1.09 grams (g) (7.78 mmol) of sodium perchlorate monohydrate in 10 milliliters (ml) of water. Air in moderation was then introduced into the reaction solution over 2 h. A red-brown solid immediately precipitated. The product was filtered off and dried in air. 2.07 g (92%) of (Co--N4Py).sub.2(.mu.-O.sub.2) was obtained as a red-brown powder.
[0099] C.sub.46H.sub.42N.sub.10Cl.sub.4Co.sub.2O.sub.10 (1154.6 g / mol)
1 Calculated C 47.85 H 3.67 N 12.13 Co 10.2 Found C 47.74 H 3.72 N 11.73 Co 9.9
example 2
[0100] Synthesis of (Co--N4Py).sub.2(.mu.-O.sub.2) by the "one-pot method"
[0101] 2.5 M aqueous NaOH solution was added to a solution of 476 mg (2.00 mmol) of CoCl.sub.2.times.H.sub.2O and 1.54 g (2.00 mmol) of N4Py-4HClO4 in 100 ml of methanol / water (1:1) until pH>7. Air in moderation was then passed through the reaction solution over 2 h. The solution was then vacuum-evaporated to half volume, the red-crystalline solid was filtered off and the latter was dried in air (820 mg, 71%).
examples 3-16
[0102] The chemicals were added to 30 g of b.d. pulp so that an aqueous solution of the additives and the catalyst was first mixed in. The pH needed for the reaction was then adjusted using NaOH. The corresponding amount of hydrogen peroxide was subsequently kneaded in and the pH was measured. The sample was then held at temperature in a polyethylene bag (PE bag) in a water bath. The results without (Comparative Example A) and with bleaching catalyst ((Co--N4Py).sub.2(.mu.-O.sub.2), (Co-MeN4Py).sub.2(.mu.-O.sub.2); Co--N4Py(CH.sub.3CN) and Fe-MeN4Py(CH.sub.3CN)) can be found in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com