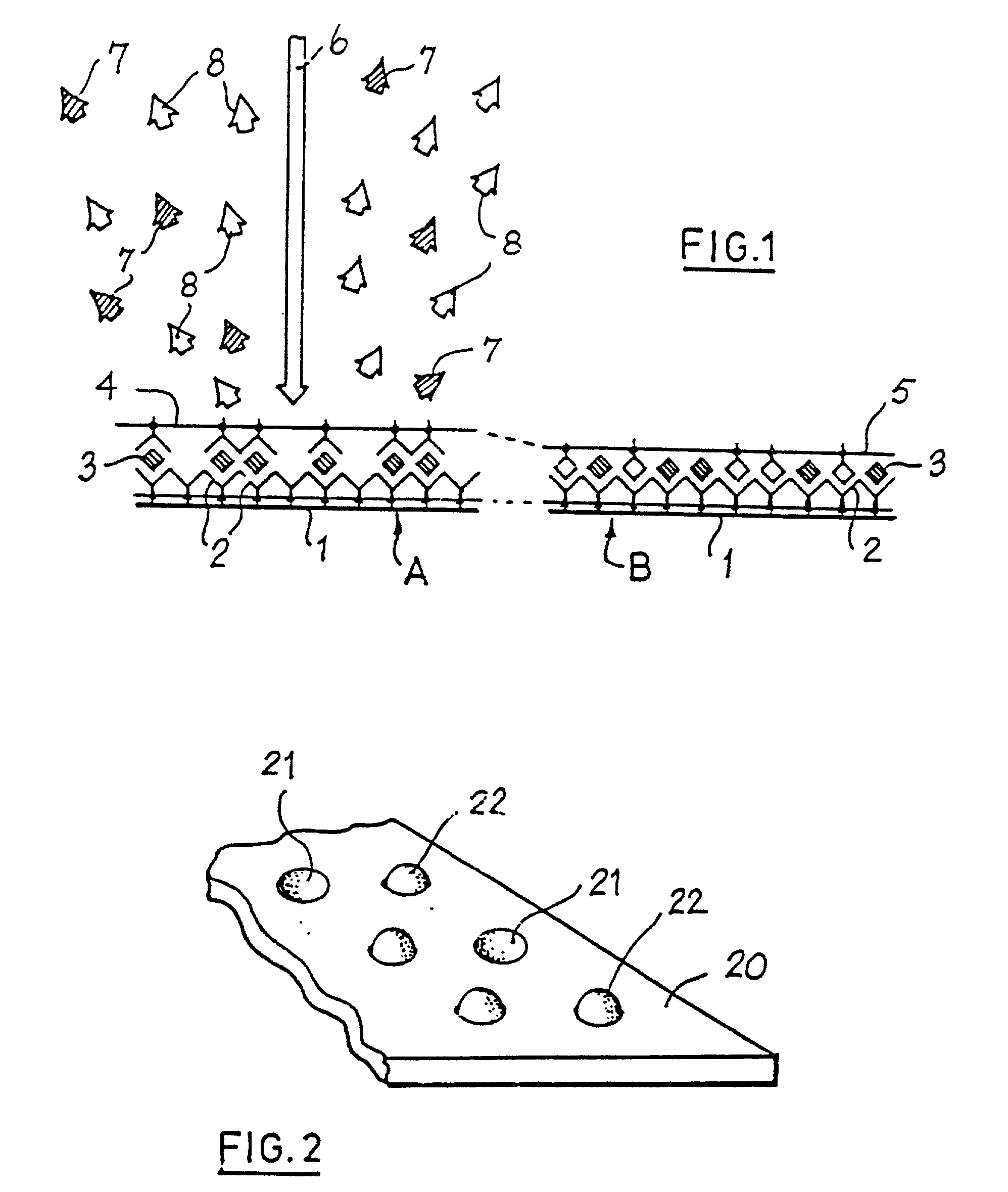
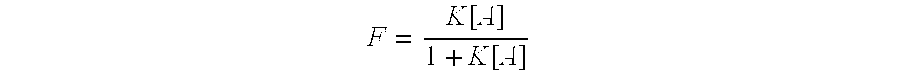Determination of analyte concentration using two labelling markers
a labeling marker and analyte technology, applied in the direction of measuring devices, instruments, material testing goods, etc., can solve the problem of negligible contribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
[0047] Establishment of a dual fluorescence T4 assay method.
[0048] Example 1 illustrates the route taken to establish a valid "dual label ratio" competitive immunoassay method. Substitution of a fluorescein label for .sup.125I for labelling T4, and of a rhodamine label for .sup.131I to label anti-T4 antibody have enabled establishment of an analogous dual-fluorescence ratio immunoassay method. The labelling of antibody and T4 with these fluorescent labels was effected by conventional methods "Immunochemistry in Practice", A Johnston and R Thorpe, Blackwell Scientific Pub (1982). However, in order to assist in selection of the correct reagent concentrations, certain experiments were conducted using dual-labelled antibody (i.e. labelled with .sup.133I and rhodamine), and dual labelled T4 (i.e. labelled with .sup.125I and fluorescein). Furthermore, antibody was coated (by adsroption) onto small polystyrene discs, rather than covalently linked to microcellulose as described in Example 1...
example 3
[0049] Establishment of a dual fluorescence T4 assay method involving time-resolved pulse fluorescence.
[0050] Instead of labelling the T4 and anti-T4 antibody with fluorescein and rhodamine as in Example 2, they were labelled respectively with terbium and europium chelates with EDTA (ethylene diamine tetraacetic acid) coupled onto the antibody in a known manner. The signal ratio *was measured by known pulsed-light fluorescence techniques using a known time-resolving fluorimeter, and the results obtained with the unknown sample compared with the calibration curve obtained with standard solutions. Again satisfactory agreement was obtained with results obtained by other methods.
example 4
[0051] A kit for use in the estimation of RSH (thyrotrophin) according to the invention is composed of the following components:
[0052] (a) A monoclonal anti-TSH antibody commercially available from the Department of Endocrinology, the Middlesex Hospital Medical School, Mortimer Street, London, is immobilised on a solid plate and labelled with fluorescein.
[0053] (b) Standard solutions contain 0.2, 1.0, 5.0, 20.0 and 100 micro-international units of TSH per mol.
[0054] (c) The back-titration reagent is likewise a commercially available anti-TSH monoclonal antibody, this time labelled with a europium (III) chelate with cupric tri-fluoroacetylacetone and formaldehyde in a manner similar to that proposed in published International Patent Application WO 86 / 01604. The first antibody is permanently fluorescent and the second is capable of estimation by time-resolved pulse fluorescence.
[0055] Such a kit can be used for the estimation of TSH by a similar procedure to that described in Examples...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com