Cell membrane directed drugs
a technology of cell membrane and drug, applied in the direction of factor vii, thrombomodulin, pharmaceutical non-active ingredients, etc., can solve the problems of increasing the use of these substances must sometimes be limited, and the side effects of the patient are sometimes serious problems, so as to achieve satisfactory effect, increase the economic burden on the patient, and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
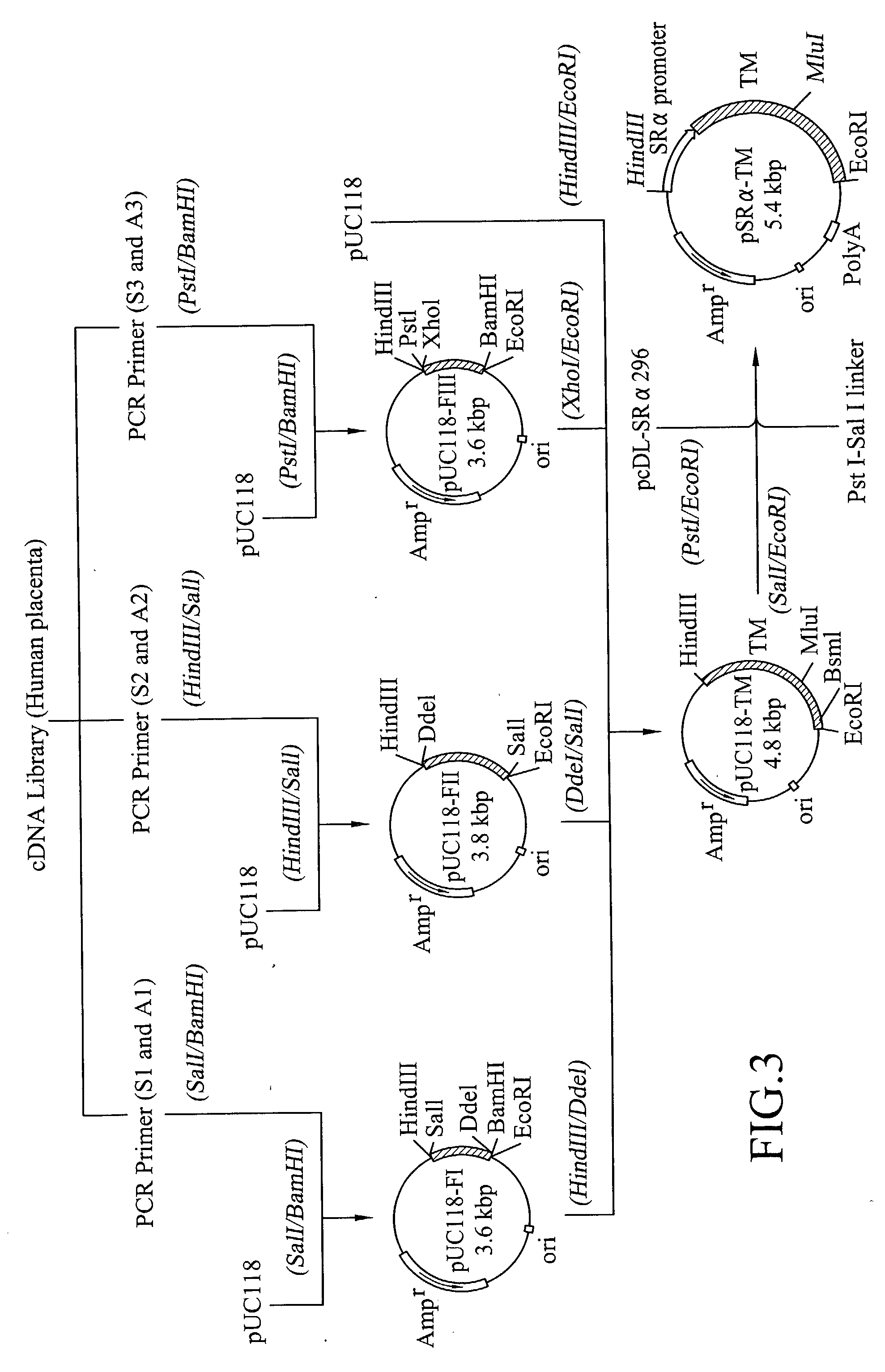
[0111] Cloning of Human TM cDNA and Preparation of Human TM Expressing Plasmids
[0112] Total RNA was isolated from about 20 g of human placenta by a guanidium isothiocyanate method. A portion (10 mg) of the resulting RNA was passed twice through a oligo(dT)-cellulose column (type 7, product of Pharmacia) to recover about 90 .mu.g of poly(A).sup.+ RNA. Then, using the resulting poly(A).sup.+ RNA as a starting material, single-stranded cDNA was synthesized. Briefly, single-stranded cDNA was synthesized by means of a reverse transcriptase using 10 .mu.g of the poly(A).sup.+ RNA as a template and an oligo(dT) as a primer in the usual manner.
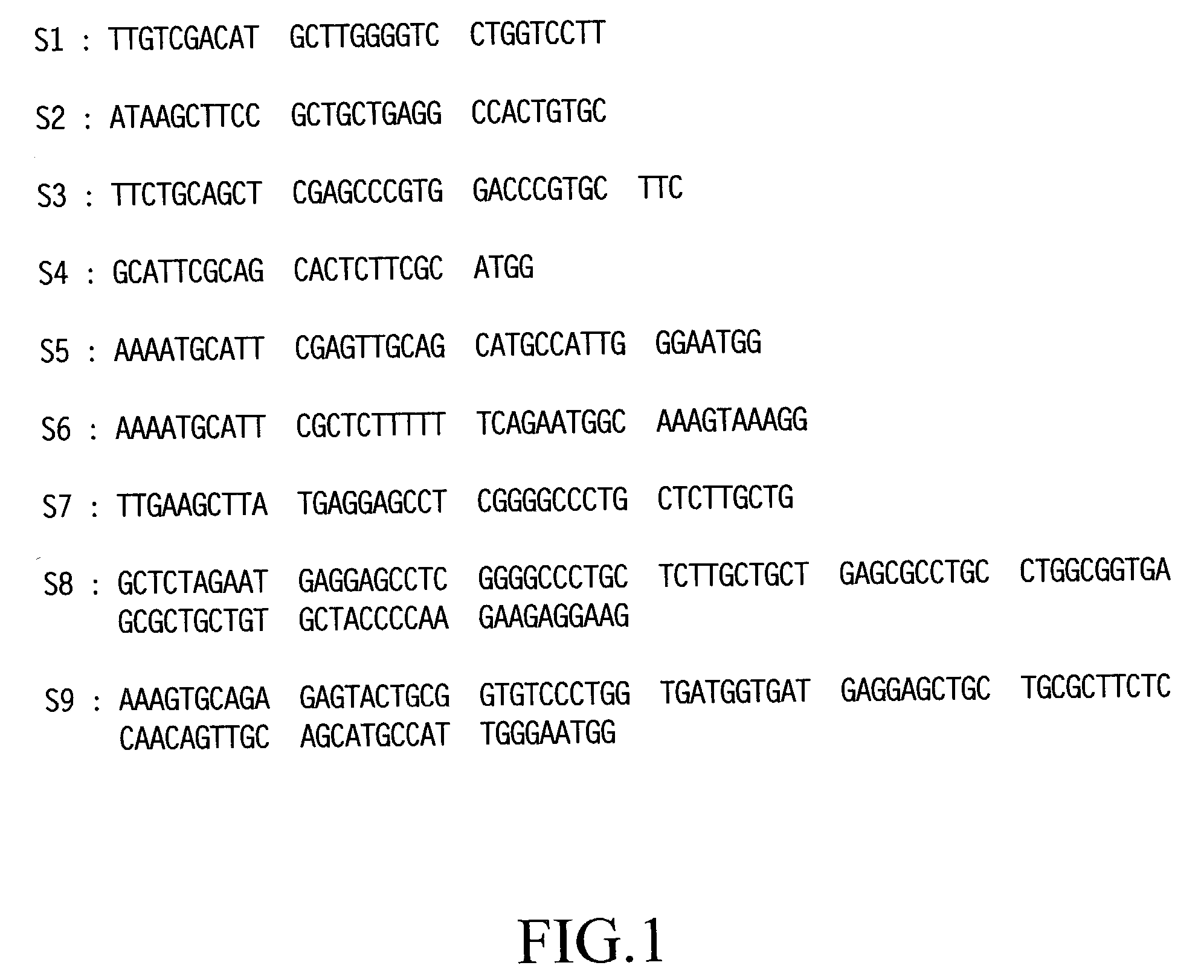
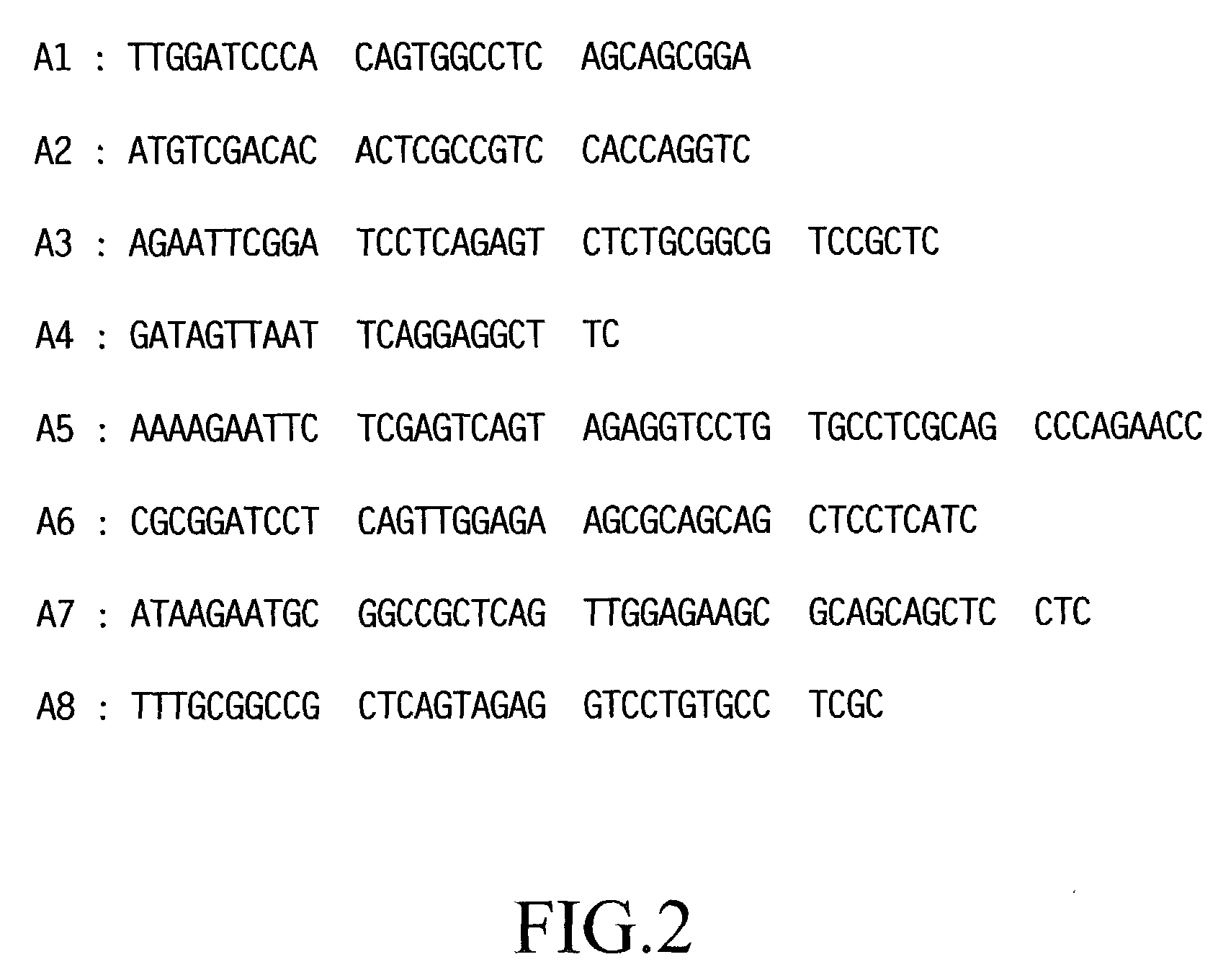
[0113] In a separate step, by referring to a known sequence of human TM DNA [K. Suzuki et al., EMBO J., Vol. 6, 1891 (1987) and R. W. Jackman et al., Proc. Natl. Acad. Sci. USA, Vol. 84, 6425 (1987)], six DNA primers (S1-S3 and A1-A3; see FIGS. 1 and 2), each corresponding to a portion of the DNA sequence of the human TM gene and containing a suitable...
example 3
[0118] Preparation of Plasmids Expressing Soluble Human TM Having Affinity for Phosphatidylserine
[0119] (1) Preparing pM1350
[0120] Four species of single-stranded DNA (F1-F4; see FIG. 4) were synthesized with a chemical synthesizer (supra) and F1 and F2 were annealed in the usual manner and so were F3 and F4, thereby yielding DNA fragment A which was ca. 30 bp in length and which had a BsmI cleaved surface at 5' end and an EcoRI cleaved surface at 3' end, as well as DNA fragment B which was ca. 90 bp in length and which had an NspV cleaved surface at 5' end and an EcoRI cleaved surface at 3' end. Then, the pUC118-TM prepared in Example 2 was digested with restriction enzymes BsmI and EcoRI and subjected to agarose gel electrophoresis, thereby separating and recovering a DNA fragment of ca. 4.6 kbp. This DNA fragment was ligated with fragment A in the usual manner to yield pUC118-TM sub1. Subsequently, the pUC118-TM sub1 was digested with restriction enzymes MluI and EcoRI and subjec...
example 4
[0137] Preparing Unmodified Soluble TM Expressing plasmid
[0138] Two species of single-stranded DNA (F11 and F12; see FIG. 5) were synthesized with a chemical synthesizer (supra) and both were annealed in the usual manner to yield DNA fragment F ca. 20 bp in length which had a BsmI cleaved surface at 5' end and an EcoRI cleaved surface at 3' end. Then, the pUC118-TM prepared in Example 2 was digested with restriction enzymes BsmI and EcoRI and subjected to agarose gel electrophoresis, thereby separating and recovering a DNA fragment of ca. 4.6 kbp. This fragment was ligated with fragment F in the usual manner to yield pUC118-sTM. Subsequently, the pUC118-sTM was digested with restriction enzymes MluI and EcoRI and subjected to agarose gel electrophoresis, thereby separating and recovering a DNA fragment of ca. 0.8 kbp. In a separate step, the pSR.alpha.-TM prepared in Example 2 was digested with restriction enzymes MluI and EcoRI and subjected to agarose gel electrophoresis, thereby ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com