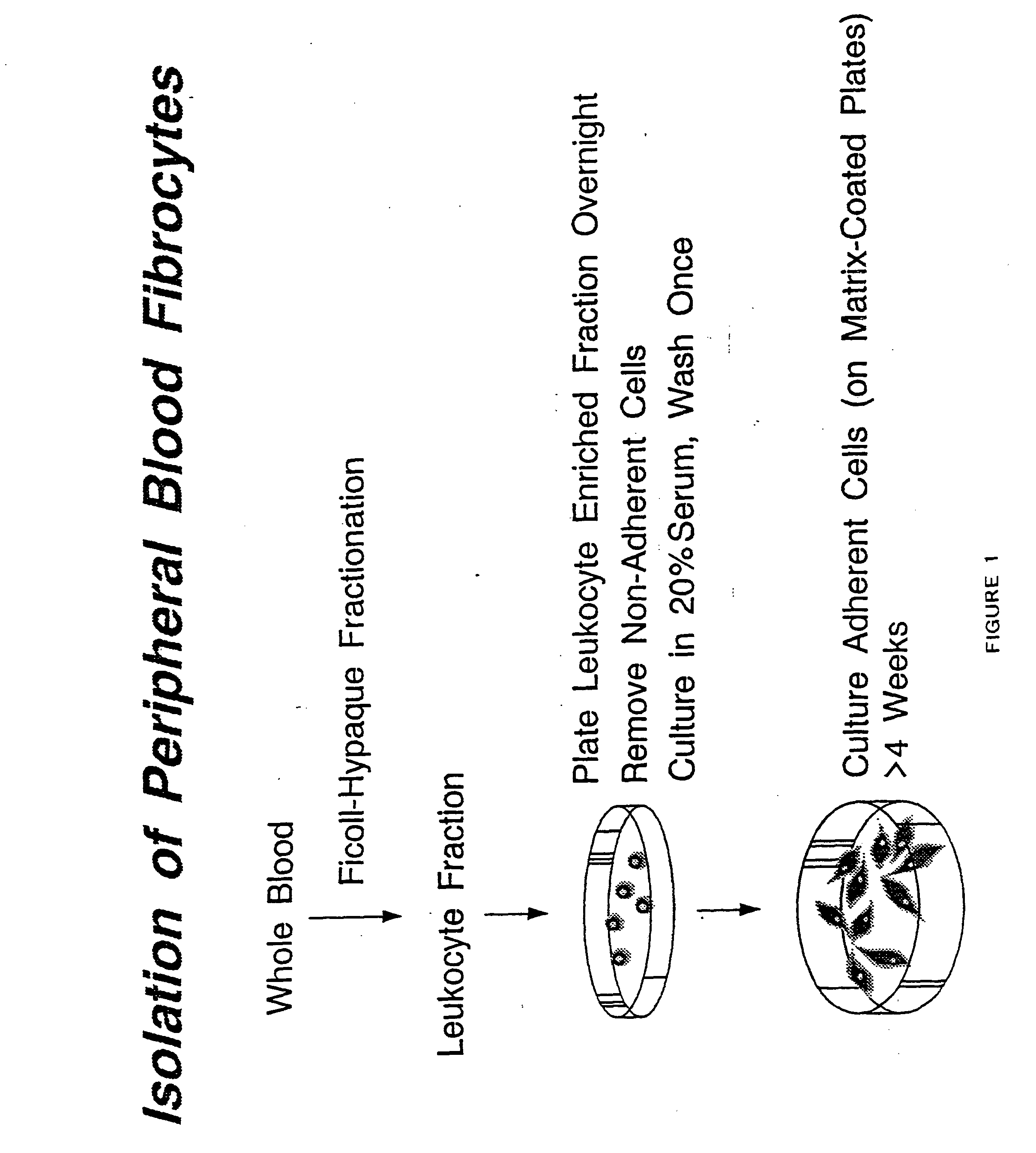
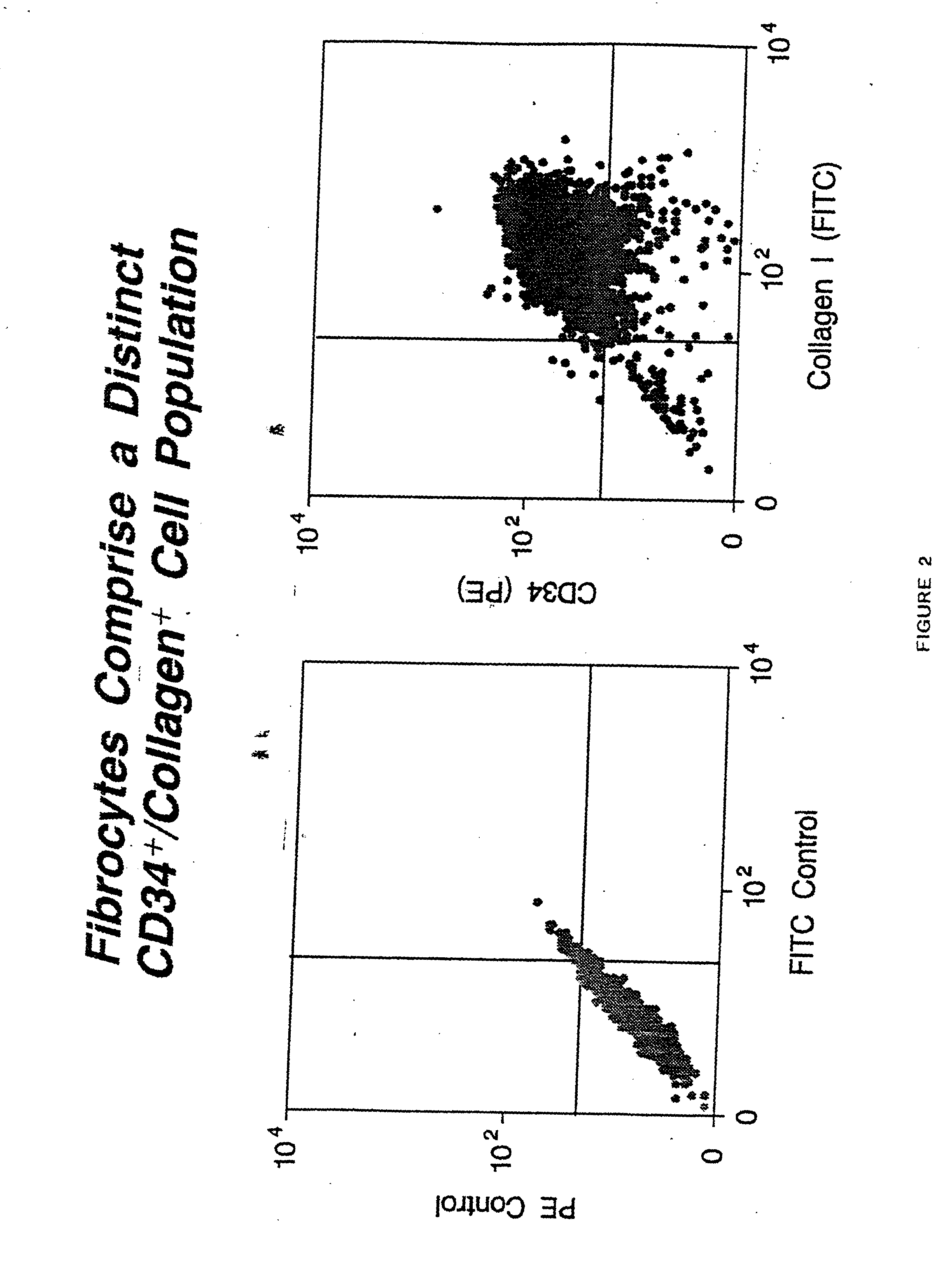
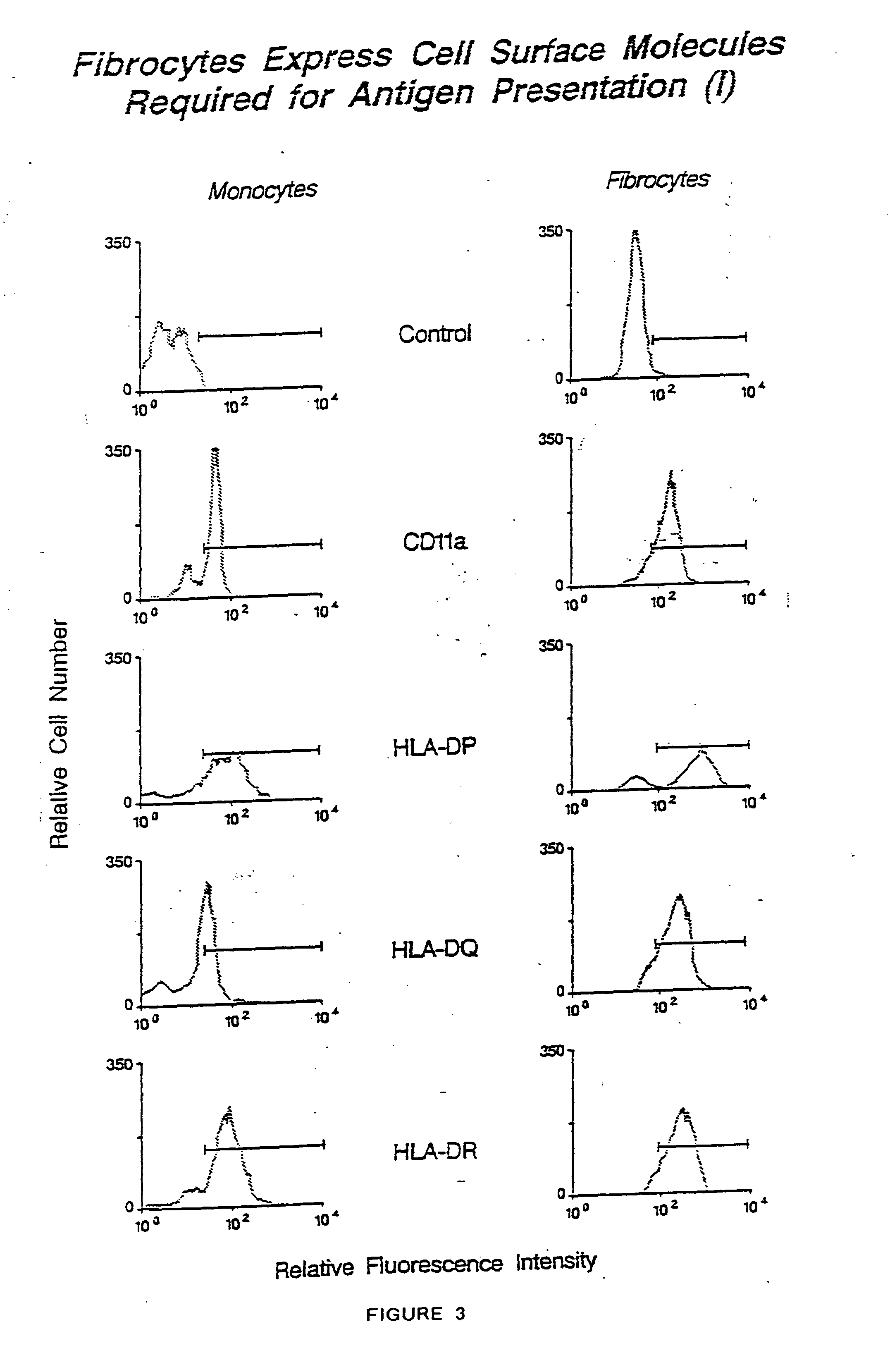
Fibrocyte-based vaccine formulations
a technology of fibrocyte and vaccine formulation, which is applied in the direction of immunological disorders, antibody medical ingredients, peptide/protein ingredients, etc., can solve the problem of insufficient first signal to activate t-cells, and achieve the effect of convenient separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
[0056] This example illustrates a comparison of the antigen presenting qualities of fibrocytes to be approximately equivalent to dendritic cells. Various concentrations of mitomycin C-treated fibrocytes were co-cultured with allogeneic T cells for 96 hours and the T cell proliferative activity assessed by the incorporation of [.sup.3H]thymidine into DNA. Fibrocytes were found to induce a significant T cell activation response (5.times.10.sup.3 fibrocytes+2.times.10.sup.5 T cells=70,321.+-.10,111 cpm, versus <300 cpm for T cells or fibrocytes alone, n=3 experiments, P<0.001).
[0057] The fibrocytes were examined for their capacity to present soluble antigen in an autologous, T cell proliferation assay. T cells were purified from the peripheral blood of tetanus toxoid-immunized individuals and stimulated with tetanus toxoid in vitro together with fibrocytes as APCs. To assess the functional capacity of fibrocytes to present antigen, we examined the ability of purified human fibrocytes t...
example 3
[0063] This example illustrates that mouse fibrocytes induced a significant antigen-dependent T cell proliferation response when co-incubated with primed, autologous T cells. T cells were purified from the peripheral blood of BALB / c mice immunized with the HIV proteins p24 and gp120 and stimulated in vitro with p24 or gp120 utilizing fibrocytes as APCs (FIG. 8). BALB / c mice were immunized by i.p. injection of 100 mg of native p24 or gp120 (purified from HIV-1.sub.IIIB infected H9 cells, Advanced Biotechnologies, Columbia, Md.) emulsified at 1 mg / ml with Freund's complete adjuvant. After 14 days, the T cells were purified from spleens by chromatography over a T cell enrichment column (R&D Systems, Minneapolis, Minn.). Mouse fibrocytes were purified from peripheral blood as described above and treated with 25 mg / ml mitomycin C (Sigma) in RPMI medium containing 10% fetal calf serum (RPMI / 10% FCS) for 30 minutes and then washed 5 times with RPMI / 10% FCS. For each assay, the T cells (2.t...
example 4
[0067] This example illustrates antigen-pulsed fibrocytes were not simply transferring antigen to other host APC types. This example provides the results of experiments in which antigen-pulsed fibrocytes from two parent mouse strains were injected into F.sub.1 offspring mice. The T cell reactivity of F.sub.1 offspring was confined predominantly to antigens presented by one of the parental strains, and the priming and re-stimulation APCs must necessarily share the same haplotype (Inaba et al., J. Exp. Med. 172:631-640, 1990; Sprent, J. Exp. Med. 147:1159, 1978; Sprent, J. Exp. Med. 147:1142, 1978). DBA-2 x C3H / HeJ F.sub.1 mice (H-2 .sup.dxk) were injected with pulsed fibrocytes from either parent (DBA-2, H-2.sup.d or C3H / HeJ, H-2.sup.k) and, five days later, the proximal popliteal lymph nodes were isolated and depleted of endogenous class II MHC.sup.+ APCs by immunomagnetic selection. The F.sub.1 APC-depleted lymph node cells were cultured with F.sub.1 (H-2.sup.dxk) or parent strain ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
| Immunogenicity | aaaaa | aaaaa |
| Antigenicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com