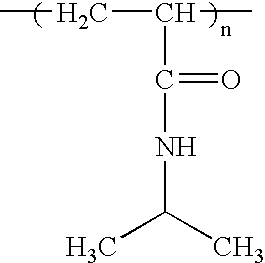
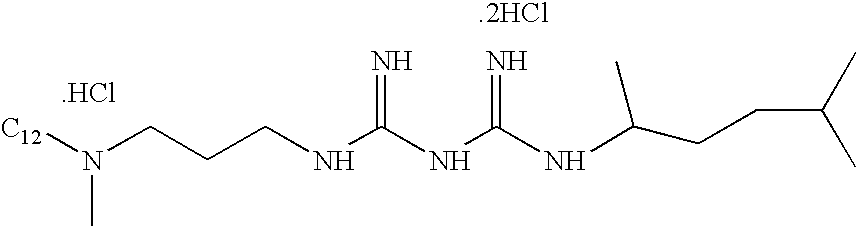
Compositions and methods for inhibiting protein on surfaces
a technology of protein adsorption and composition, applied in the direction of detergent compounding agents, lens cleaning compositions, drug compositions, etc., can solve the problems of microbial contamination, support, and other devices or objects that can be adversely affected by protein adsorption, and achieve the effect of preventing microbial contamination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
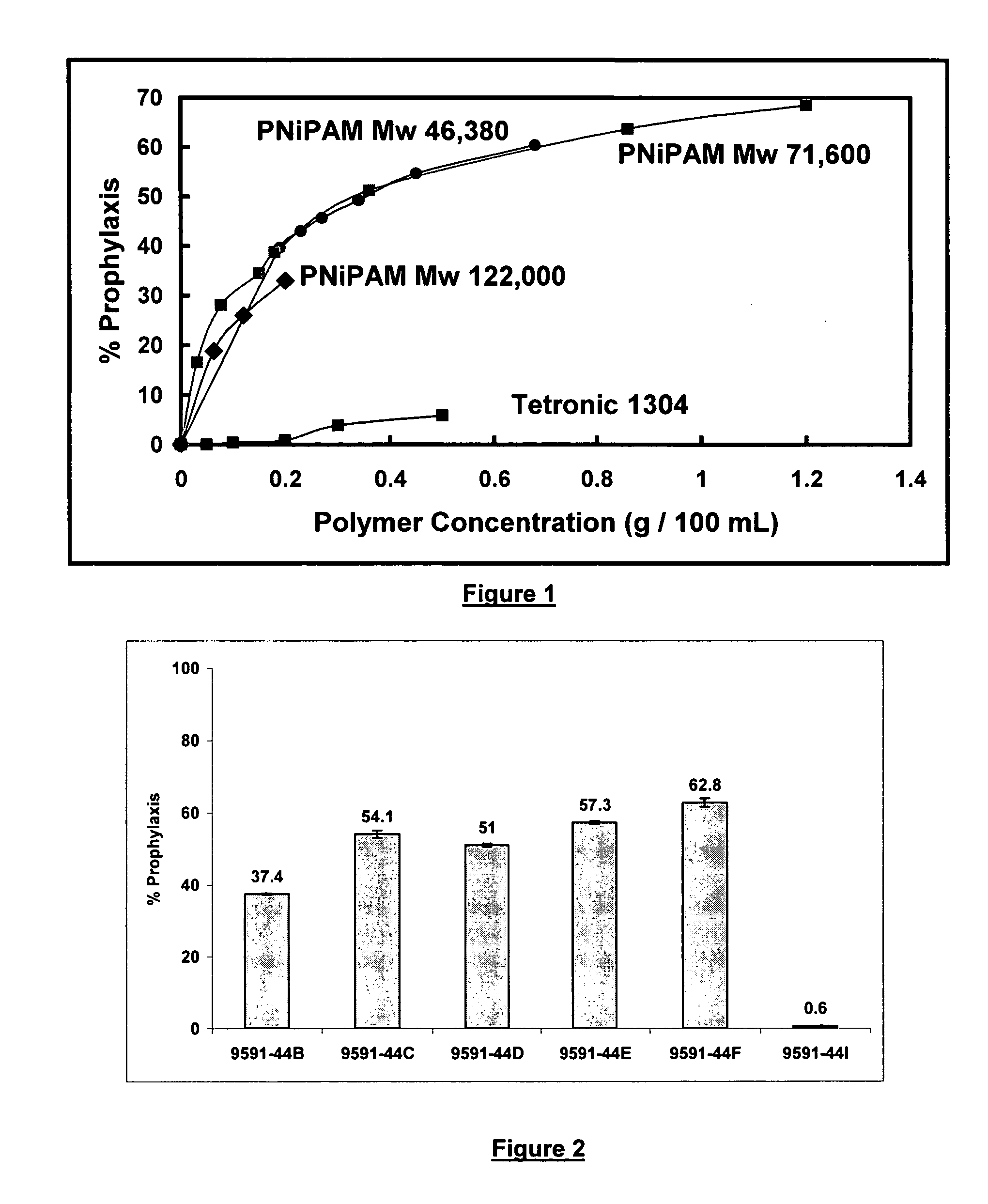
[0071] The prophylactic properties of NIPAM polymers were further evaluated using a 3-day cycling study. Two sets of lenses were prepared. One set was presoaked in the formulations shown in Table 2 before going into the lysozyme solution, whereas the other set was not. Both sets of lenses were then placed in the lysozyme solution for 8 hours (Day 1). At the end of the day all the lenses were rinsed and put in their respective formulations to soak overnight. The following day (Day 2), the lenses went back into the lysozyme for the day (8 hours). This was repeated to complete 3 cycles (3 Days). At the end of the experiment all the lenses were analyzed in accordance with the procedures described in Example 1. The results are presented in Table 3:
3TABLE 3 Uptake Amount of Lysozyme Removed Sample (ug / lens) sd (ug / lens) % Prophylaxis sd 9591-47A(PS) 124.1 9.1 261.9 67.8 0.8 9591-47B(PS) 151.5 3.9 234.5 60.8 0.6 9591-47C(PS) 386.0 6.1 -- -- --9591-47A 206.3 2.7 174.9 45.9 1.2 9591-47B 221....
example 3
[0074] The prophylaxis work was extended to formulations containing the antimicrobial agent AL-8496 with unmodified NIPAM (non-ionic) and modified NIPAM (end functionalized with COOH) polymers. The formulations evaluated are shown in Table 4, below:
4TABLE 4 Formulations for Microbiology Evaluation of PNIPAM Formulations Containing A Contact Lens Disinfecting Agent (AL-8496) Formulation Numbers 9591-44I Component 9591-44B 9591-44C 9591-44D 9591-44E 9591-44F (Control) P2991-NIPAM 0.087 0.21 P2426F2- 0.040 0.10 0.25 NIPAMCOOH AL-8496* 0.0004 0.0004 0.0004 0.0004 0.0004 0.0004 Tetronic .RTM. 1304 0.1 0.1 0.1 0.1 0.1 0.1 Sorbitol 0.4 0.4 0.4 0.4 0.4 0.4 Sodium borate 0.2 0.2 0.2 0.2 0.2 0.2 Sodium citrate 0.6 0.6 0.6 0.6 0.6 0.6 Propylene glycol 1.0 1.0 1.0 1.0 1.0 1.0 Disodium edetate 0.05 0.05 0.05 0.05 0.05 0.05 pH 7.8 7.8 7.8 7.8 7.8 7.8 % Prophylaxis 37.4 .+-. 0.2 54.1 .+-. 1.0 51.0 .+-. 0.5 57.3 .+-. 0.4 62.8 .+-. 1.2 0.6 .+-. 0.0 *As base
[0075] The procedures utilized were the sam...
example 4
[0077] The disinfection activity of the formulations shown in Table 4 above was also evaluated. The results are shown in Table 5 below.
5TABLE 5 Disinfection Properties of PNIPAM Formulations containing AL-8496 Time 9591- 9591- 9591-9591- 9591- 9591-Microorganism (hrs) 44B 44C 44D 44E 44F 44I Candida 6 2.8 3.0 3.0 3.4 3.2 3.0 albicans 24 3.9 4.5 6.0 6.0 5.3 6.0 Serratia 6 2.7 6.2 2.8 2.7 2.6 2.6 marcescens 24 5.5 6.2 5.5 6.2 5.5 4.9 Staphylococcus 6 5.5 4.5 5.5 4.4 4.3 4.9 aureus 24 6.2 5.0 6.2 6.2 6.2 5.2
[0078] The results demonstrate that the NIPAM polymers did not adversely affect the antimicrobial activity of the antimicrobial agent AL-8496.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weights | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com