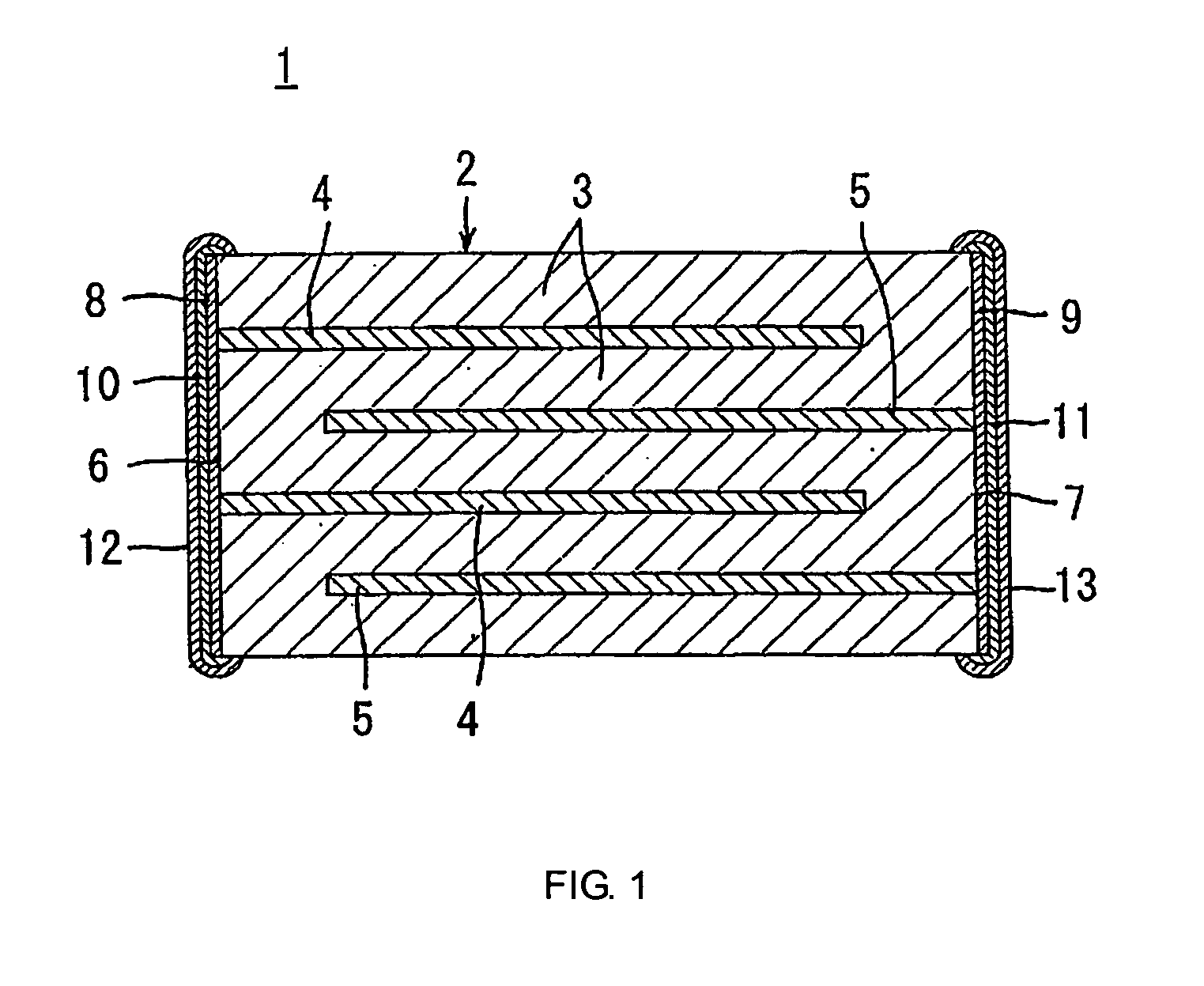
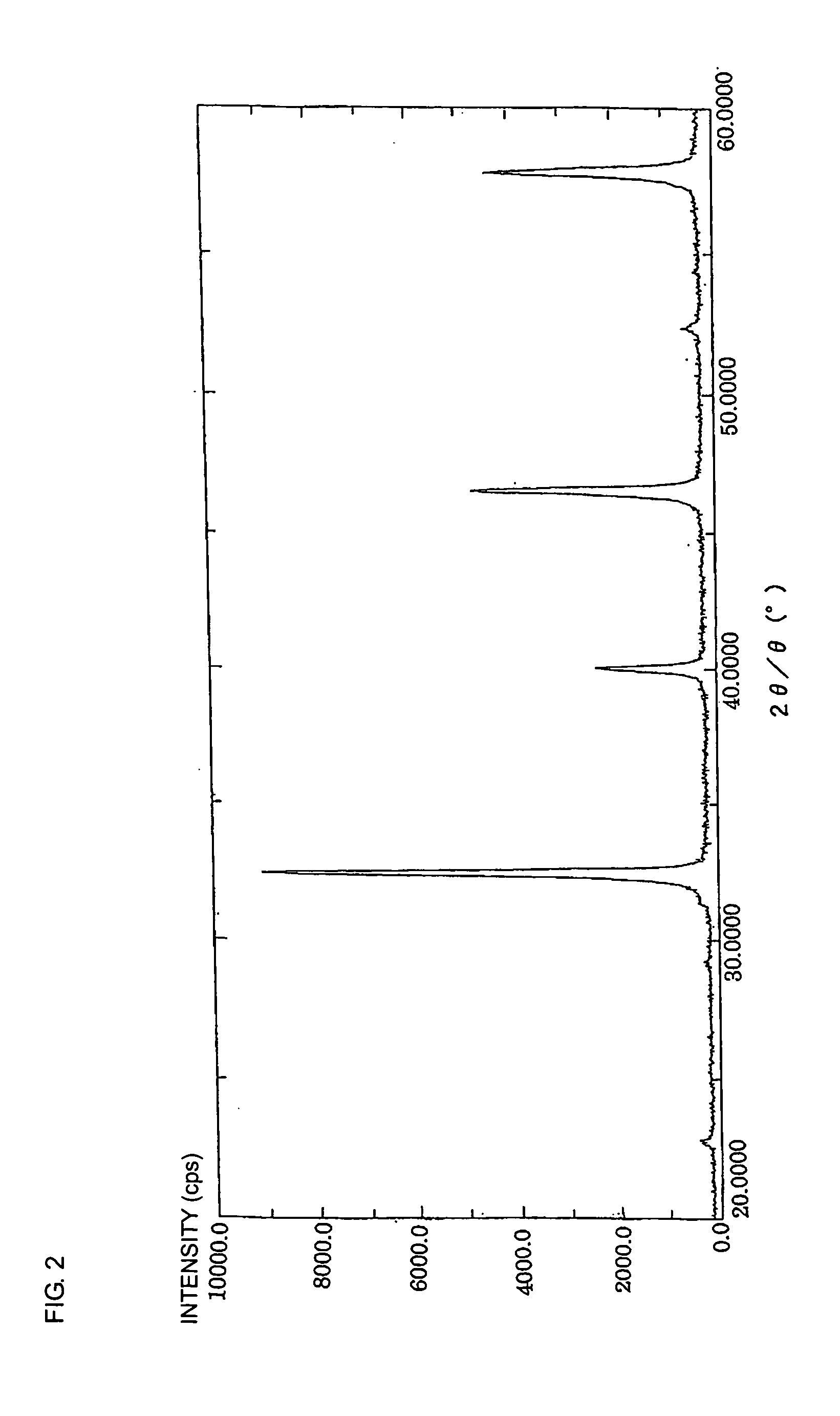
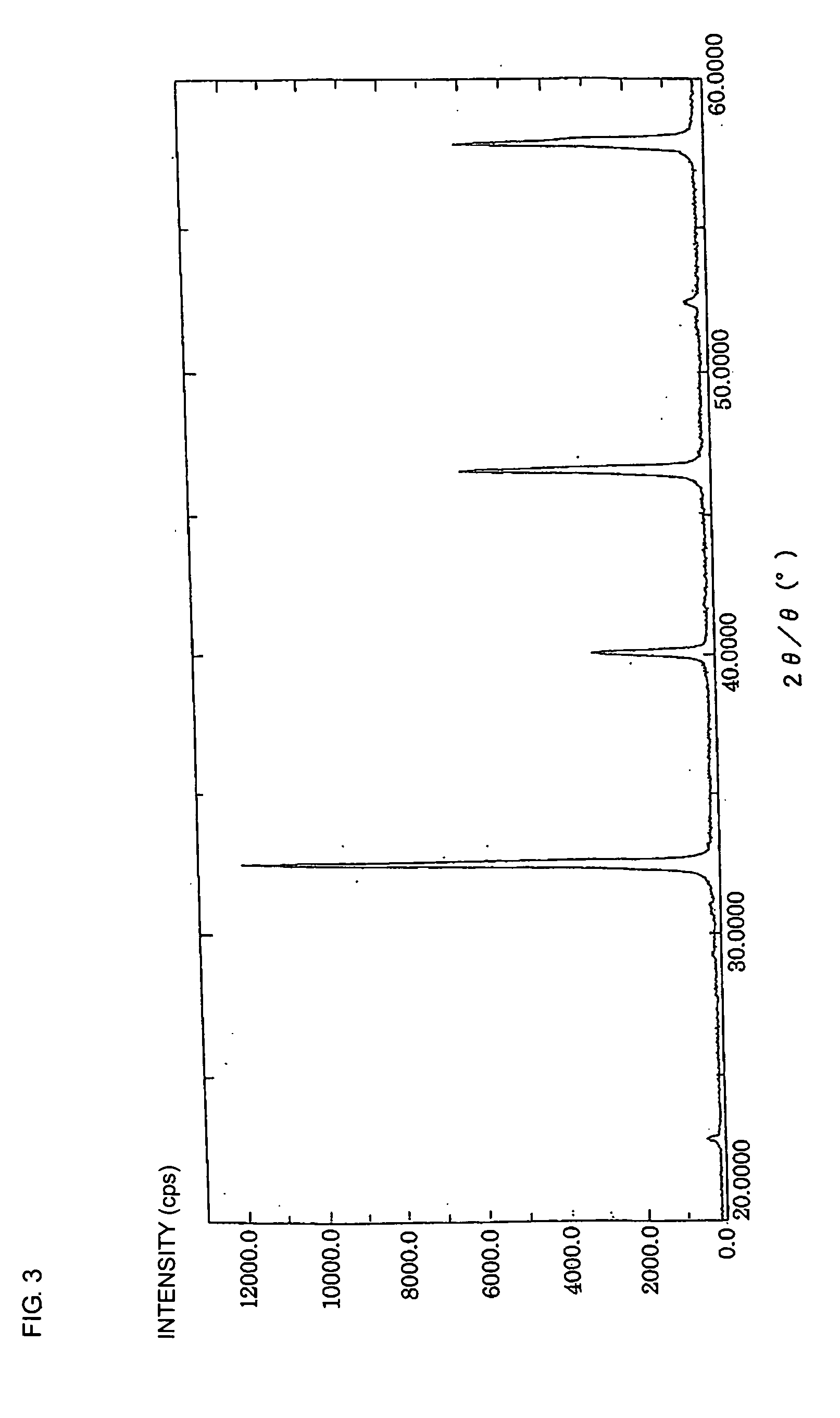
Nonreducing dielectric ceramic, its production method and multilayer ceramic capacitor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
experimental example 2
[0121] Prepared were SrCO.sub.3, CaCO.sub.3, BaCO.sub.3, ZrO.sub.2, TiO.sub.2 and HfO.sub.2 powders with purities of 99% or more as the starting powder materials for the principal constituents, as in Experimental Example 1. Oxide powders of Dy, Ho, Er, Tm, Yb, Lu, Y, and Sc were prepared as a starting material for accessory constituent 1. Also, NiO, MnCO.sub.3, and COCO.sub.3 powders were prepared as a starting powder material for accessory constituent 2, as in the accessory constituents in Experimental Example 1.
[0122] The starting powder materials for the principal constituents were weighed out so as to constitute the composition expressed by the formula
(Sr.sub.1-w-xCa.sub.wBa.sub.x).sub.m(Ti.sub.1-y-zZr.sub.yHf.sub.z)O.sub.3,
[0123] according to the values of w, x, y, z, and m shown in Table 4. Also, the starting powder materials for accessory constituents 1 and 2 were weighed out so that their mole numbers became the values shown in Table 4 relative to 100 moles of the principal ...
experimental example 3
[0150] SrCO.sub.3, CaCO.sub.3, and TiO.sub.2 powders with purities of 99% or more were prepared as the starting powder materials for the principal constituents. Also, Yb.sub.2O.sub.3, MnO, Al.sub.2O.sub.3, and MgCO.sub.3 powders were prepared as the starting powder materials for the accessory constituents. These starting powder materials were each weighed out so that the principal constituents form the composition (Sr.sub.0.6Ca.sub.0.4)TiO.sub.3 and that the mole numbers of the accessory constituents in terms of YbO.sub.3 / 2, MnO, AlO.sub.3 / 2, or MgCO.sub.3 became the values shown in Table 7 relative to 100 moles of the principal constituents. The weighed starting powder materials were wet-mixed to prepare an uncalcined powder mixture in the same manner as in Experimental Example 1.
7TABLE 7 Sample YbO.sub.3 / 2 MnO AlO.sub.3 / 2 MgCO.sub.3 number (mol) (mol) (mol) (mol) 58 3.0 2.0 -- --59 3.0 0.5 2.5 --60 3.0 1.0 4.0 --61 3.0 2.0 5.5 --62 3.0 0.5 -- 1.5 63 3.0 1.0 -- 2.5 64 3.0 2.0 -- 4....
experimental example 4
[0161] Prepared were SrCO.sub.3, CaCO.sub.3, ZrO.sub.2, and TiO.sub.2 powders with purities of 99% or more as the starting powder materials for the principal constituents. Also, Yb.sub.2O.sub.3 and MgCO.sub.3 powders were prepared as the starting powder materials for the accessory constituents.
[0162] These starting powder materials were each weighed out so that the principal constituents form the composition (Sr.sub.0.8Ca.sub.0.2) (Ti.sub.0.8Zr.sub.0.2)O.sub.3 and that the mole numbers of the accessory constituents Yb.sub.2O.sub.3 and MgCO.sub.3 in terms of YbO.sub.3 / 2 or MgCO.sub.3 each became 3.0 moles relative to 100 moles of the principal constituents. The weighed starting powder materials were wet-mixed to prepare an uncalcined powder mixture in the same manner as in Experimental Example 1.
[0163] The uncalcined powder mixture was preliminarily calcined at 800.degree. C. for 2 hours in the air to prepare a preliminarily calcined material.
[0164] After being mixed and pulverized i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com