Methods of oxidizing multiwalled carbon nanotubes
a carbon nanotube and multi-walled technology, applied in carbon nanotubes, inorganic chemistry, electrolytic capacitors, etc., can solve the problems of graphite and carbon black poor predictors of nanotube chemistry, fibrils have also been oxidized non-uniformly, and no practical significance, etc., to achieve enhanced electrochemical characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Oxidation of Carbon Nanotubes With Gas Phase CO2
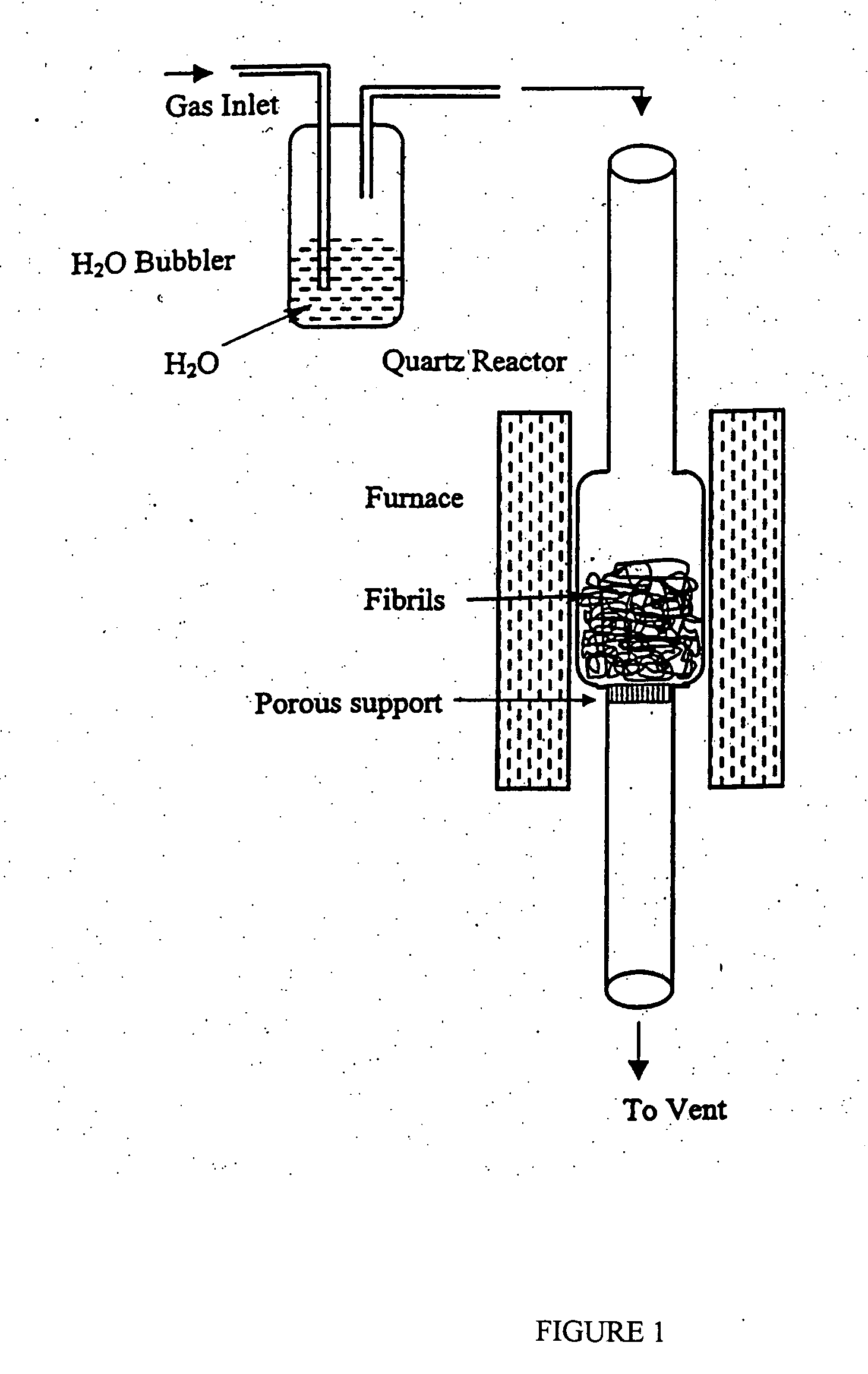
[0150] Oxidized carbon nanotubes were prepared by using CO2 in the gaseous phase. About 10 grams of carbon nanotubes were placed into a reactor as shown in FIG. 1. The reactor was a heated quartz tube having a reacting chamber connected at each end to a side tube. The reacting chamber had an outside diameter of about 3 inches and each side tube has an outside diameter of about 1 inch. Between the side tube at the bottom side and the reacting chamber there was a gas permeable porous quartz plate, which supports a bed of carbon nanotubes prepared as described in U.S. application Ser. No. 08 / 459,534 filed on Jun. 2, 1995.
[0151] A stream of gaseous CO2 was continuously passed down through the bed of carbon nanotubes at a rate of about 120 cc / min for 2 hours at about 800° C.
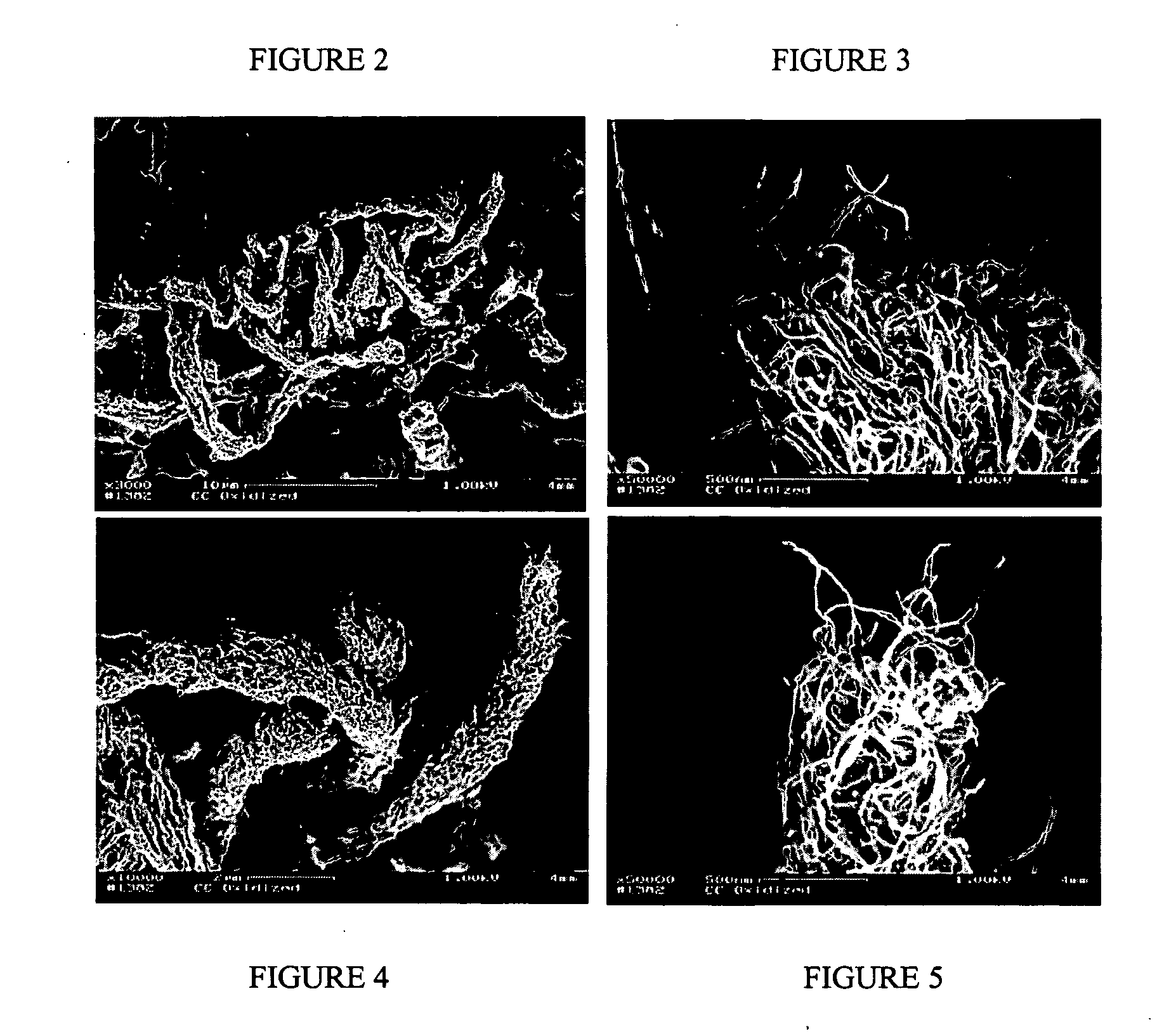
[0152] The degree of oxidation was measured by the weight loss exhibited by the carbon nanotubes; a weight loss of about 10% was recorded. The carbon nanotubes oxidiz...
example 2
Oxidation of Carbon Nanotubes With Wet-air
[0153] Carbon nanotubes were oxidized by using wet air. About 10 grams of carbon nanotubes prepared according to U.S. application Ser. No. 08 / 459,534 filed on Jun. 2, 1995 were charged into the reactor described in Example 1.
[0154] Air saturated with water vapor at room temperature was continuously passed down through the bed of carbon nanotubes at a rate of about 120 cc / min. The temperature of the reactor, measured by a k-type thermocouple positioned inside the bed of carbon nanotubes, was set at 530° C. The degree of oxidation was controlled by variation of the reaction duration and monitored by weight loss, compared to the initial weighted unoxidized carbon nanotubes. Three samples with weight losses of 7.1, 12.4, and 68% corresponding to 4, 5, and 8 hr oxidation, respectively, were prepared.
example 3
Oxidation of Carbon Nanotubes With Oxygen
[0155] Carbon nanotubes are oxidized by using oxygen in the gas phase. About 10 grams of carbon nanotubes prepared according to U.S. Ser. No. 08 / 459,534 filed on Jun. 2, 1995 are charged into a reactor as described in Example 1.
[0156] A stream of gaseous oxygen is continuously passed down through the bed of carbon nanotubes at a rate of about 120 cc / min for 2 hours at 600° C. The temperature of the reactor is measured by a k-type thermocouple positioned inside the bed of carbon nanotubes. The degree of oxidation is controlled by variation of the reaction duration and monitored by weight loss as compared to the initial weight of unoxidized carbon nanotubes. The resulting weight loss is about 10%. The carbon nanotubes oxidized in this manner disperse in water quite easily whereas they hardly do so prior to treatment with gaseous oxygen.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com