Inhibitors of serine proteases, particularly HCV NS3-NS4A protease
a protease and serine technology, applied in the field of serine proteases, can solve the problems of inability to broadly effective treat the debilitating progression of chronic hcv, inability to use interferons, and inability to achieve satisfactory anti-hcv agents or treatments, so as to reduce the risk of or severity of hcv infection, inhibit hcv replication, and improve the effect of anti-hcv
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiments
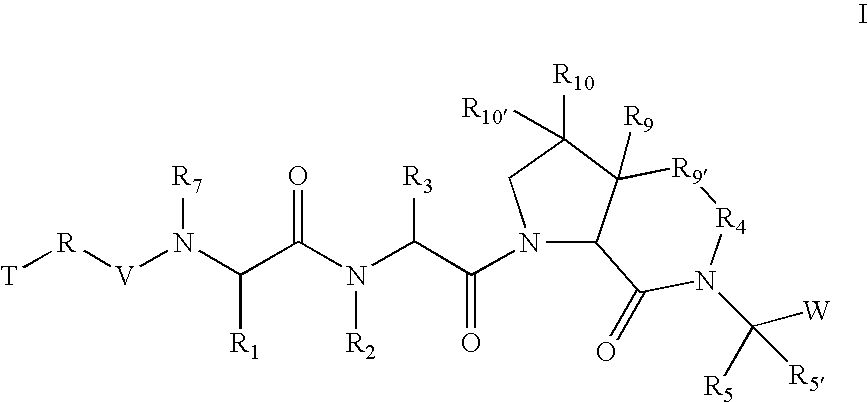
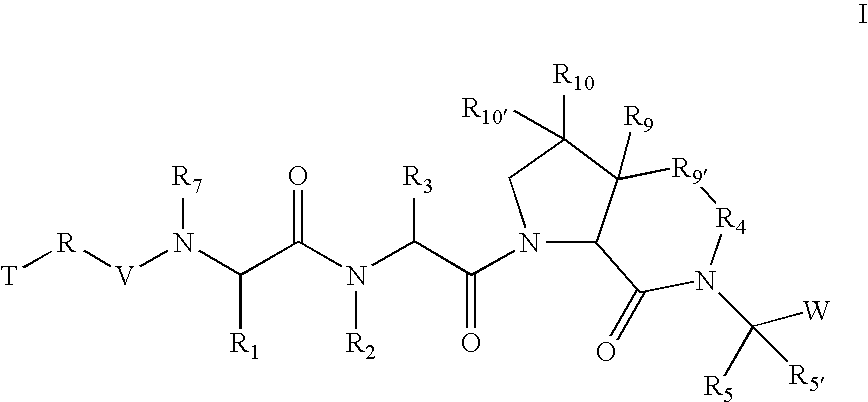
[0261] According to one embodiment of compounds of formula I, the
radical is,
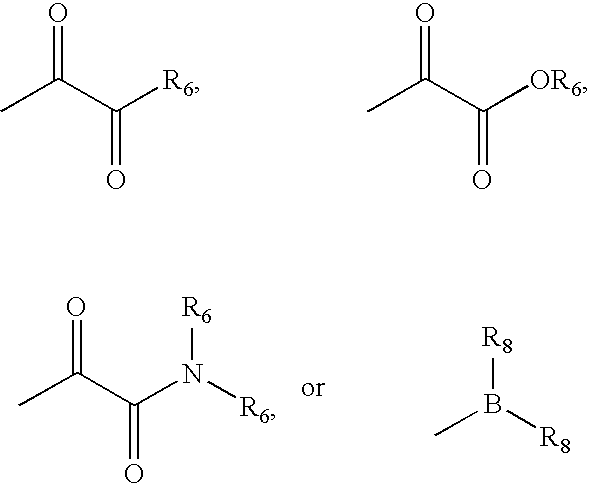
wherein; [0262] in R9, R10, and R10′, X and Y are both a bond and Z is hydrogen; and in R9′; [0263] X is a bond; [0264] Y is a bond, —CH2—, or —C(O)—; and [0265] Z is (C1-C12)-aliphatic-, [0266] (C3-C10)-cycloalkyl- or -cycloalkenyl-, [0267] [(C3-C10)-cycloalkyl or -cycloalkenyl]-(C1-C12)-aliphatic-, [0268] (C6-C10)-aryl-, [0269] (C6-C10)-aryl-(C1-C12)aliphatic-, [0270] (C3-C10)-heterocyclyl-, [0271] (C3-C10)-heterocyclyl-(C1-C12)aliphatic-, [0272] (C5-C10)-heteroaryl-, or [0273] (C5-C10)-heteroaryl-(C1-C12)-aliphatic-; [0274] wherein up to three aliphatic carbon atoms in Z may be optionally replaced with S, —S(O)—, —S(O)—, —O—, —N—, or —N(H)—, in a chemically stable arrangement; [0275] wherein any ring may be optionally fused to a (C6-C10)aryl, (C5-C10)heteroaryl, (C3-C10)cycloalkyl, or (C3-C10)heterocyclyl; [0276] wherein Z may be independently and optionally substituted with up to 3 substituents ind...
example 1
Pyrazine-2-carboxylic acid (cyclohexyl-{1-[2-(1-cyclopropylaminooxalyl-butylcarbamoyl)-3-isopropyl-pyrrolidine-1-carbonyl]-2,2-dimethyl-propylcarbamoyl}-methyl)-amide (56)
[0520] To a stirring suspension of copper bromide-dimethylsulfide (9.1 g, 44.28 mmol) in 100 mL of dry ether at −20° C. was added isopropenyl magnesium bromide 0.09M (100 mL). After 15 min. of stirring, the temperature was lowered to −78° C. and enone 4a (4.0 g, 8.86 mmol, prepared according to procedure in JACS, 117, p. 10775, (1995)) in 50 mL of ether was added followed by TMSCl (2.25 mL, 18 mmol). The reaction mixture was stirred at −78° C. for 1 h and quenched with 100 mL of ammonium hydroxide-ammonium chloride solution (1:4). Extracted with ether and the organic phase was washed to remove all the copper salts. The ether layer was dried with sodium sulfate and concentrated in vacuo to an oil that was subjected to flash chromatography (ether-hexanes (2:3) to provide 3.5 g (73%) of the desired intermediate olefi...
example 2
3-tert-Butyl-2-(tert-butyl-dimethyl-silanyloxymethyl)-5-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester (6a)
[0532] t-Butyl zinc bromide 0.5M solution in THF(3.7 mL, 1.83 mmol) was added to a solution of enone 4a (280 mg, 0.85 mmol) in THF containing BF3OEt2 (350 uL, 2.75 mmol) and TMSCl (465 uL) at −30° C. over a period of 5 minutes. The heterogeneous mixture was stirred at −30° C. for 3.5 h than quenched with sat'd NH4Cl solution. Extracted with ether (3×) and the combined extract were washed with brine, dried with sodium sulfate and concentrated in vacuo. Flash chromatography (10% ethyl acetate-hexanes) provided 210 mg (64%) of 6a. 1H NMR (CDCl3) d 3.9 (s, 1H); 3.8 (dd, 1H); 3.5 (d, 1H); 2.8 (dd, 1H); 2.3 (d, 1H); 1.9 (d, 1H); 1.4 (s, 9H); 0.9 (s, 18H); 0.1 (s, 3H); 0.05 (s, 3H) ppm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com