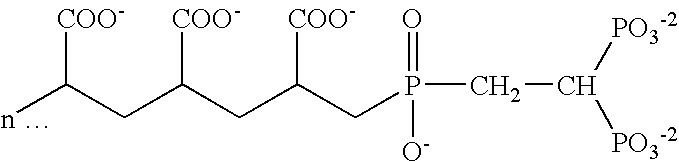
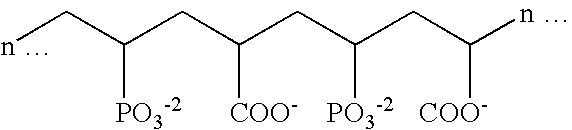
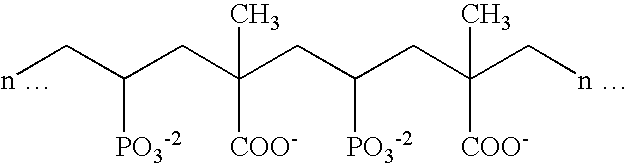
Stannous oral compositions
a technology of stannous fluoride and composition, which is applied in the field of improved oral compositions, can solve the problems of increasing or improving the effectiveness of the formulation, increasing or improving the side effects of the formulation, and complicated commercial utilization, and achieves the effects of astringency, reducing the side effects of tooth staining, and ensuring the effect of oral health
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples &
METHOD OF MANUFACTURING
[0088] The following examples and descriptions further clarify embodiments within the scope of the present invention. These examples are given solely for the purpose of illustration and are not to be construed as limitations of the present invention as many variations thereof are possible without departing from the spirit and scope.
example i
[0089] Example I illustrates dual phase dentifrice compositions incorporating sodium polyphosphate (Glass H supplied by FMC Corporation, n=21 unit condensed phosphate polymer) in the First Dentifrice composition and incorporating stannous fluoride and other stannous salts in the Second Dentifrice composition.
First Dentifrice CompositionsFormulaFormulaFormulaFormulaIngredient1a2a3a4aCarboxymethycellulose0.5000.2000.2000.300Water2.210——1.400Flavor1.5001.1001.1001.100Glycerin29.890 45.550 43.550 39.850 Polyethylene Glycol1.500——6.000Polyoxyethylene0.200———Propylene Glycol8.000———Sodium Lauryl Sulfate(a)10.000 8.00010.000 6.000Silica15.000 18.150 18.150 26.000 Polyoxyl 40 Hydrogenated2.500——Castor OilBenzoic Acid0.600——0.300Sodium Benzoate0.600——0.300Sodium Saccharin0.4000.4000.4000.350Titanium Dioxide1.0000.5000.5000.400Glass H Polyphosphate25.800 26.000 26.000 18.000 Xanthan Gum0.3000.1000.100—Second Dentifrice CompositionsFormulaFormulaFormulaFormulaIngredient1b2b3b4bPolyoxyethylen...
example ii
[0093] Example II illustrates single phase dentifrice compositions incorporating stannous ion salts, dispersions of suspended Glass H polyphosphates or polyphosphonate polymer and various fluoride ion sources all formulated within low water base to facilitate polymeric MSA stability and stannous ion stability. Also shown is an example of a stannous-containing dentifrice as described in U.S. Pat. No. 5,004,597 to Majeti et al.
[0094] Example II compositions (Formula A-D below) are prepared as follows. Add the glycerin and thickening agents to the main mix tank and mix until homogeneous. If applicable, add the sodium gluconate to the main mix tank and mix until homogeneous. Add the sodium lauryl sulfate solution and flavor to the main mix tank and mix until thickeners are hydrated / dissolved. Add the silica and titanium dioxide to the main mix tank and mix until homogeneous. Add stannous and / or fluoride salts to the main mix tank and mix until homogeneous. Finally add the polymeric sur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molar ratio | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com