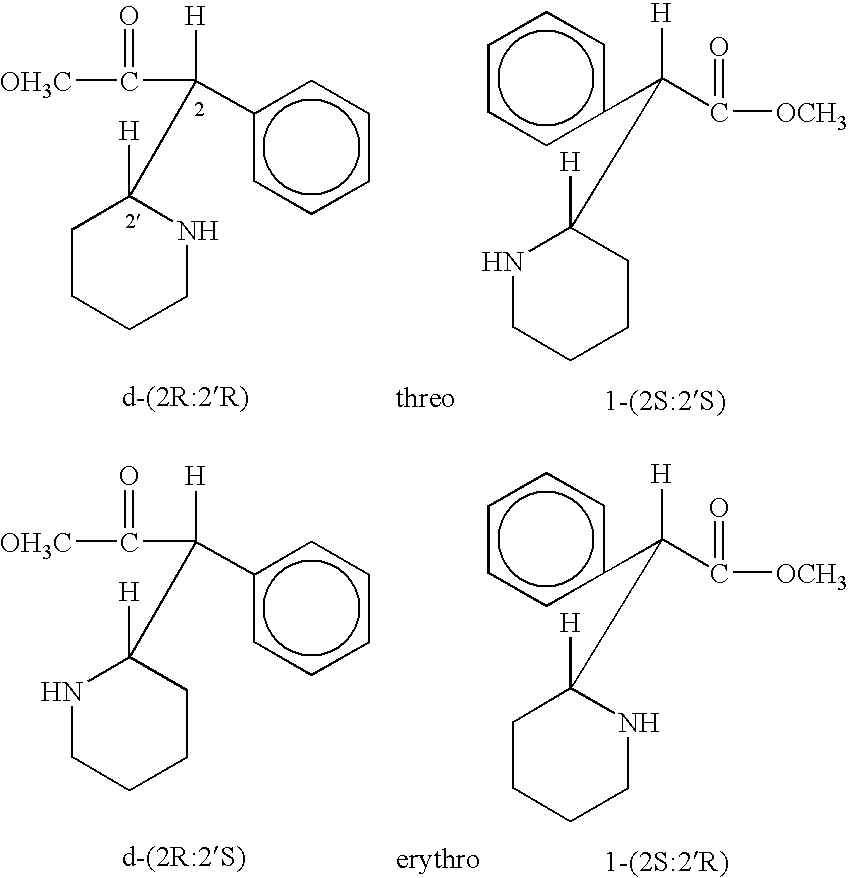
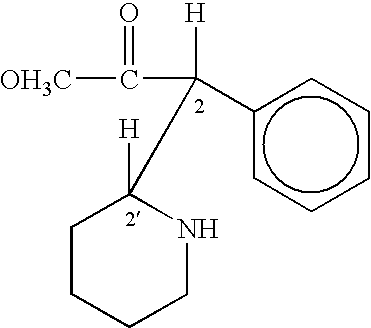
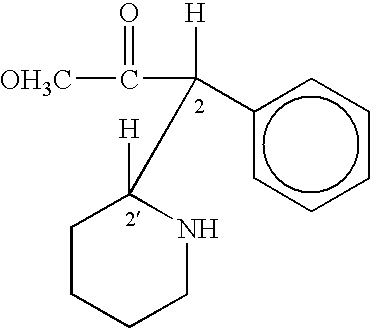
Method of treating attention deficit disorders with D-threo methylphenidate
a technology of dthreomethylphenidate and attention deficit disorders, which is applied in the direction of heterocyclic compound active ingredients, biocide, drug compositions, etc., can solve the problems of high potential for substance abuse in patients, and high potential for drug abuse in patients, so as to improve cognitive function, reduce side effects, and enhance therapeutic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0032] Tablets for chewing, each containing 5 milligrams of d-threo-methylphenidate, can be prepared in the following manner:
Composition (for 1000 tablets)d-threo-methylphenidate 5.00 gramsmannitol15.33 gramslactose10.00 gramstalc 1.40 gramsglycine 0.83 gramsstearic acid 0.66 gramssaccharin 0.10 grams5% gelatin solution q.s.
[0033] All the solid ingredients are first forced through a sieve of 0.25 mm mesh width. The mannitol and the lactose are mixed, granulated with the addition of gelatin solution, forced through a sieve of 2 mm mesh width, dried at 50° C. and again forced through a sieve of 1.7 mm mesh width. The d-threo-methylphenidate, the glycine and the saccharin are carefully mixed, the mannitol, the lactose granulate, the stearic acid and the talc are added and the whole is mixed thoroughly and compressed to form tablets of approximately 10 mm diameter which are concave on both sides and have a breaking groove on the upper side.
example 2
[0034] Tablets, each containing 10 milligrams of d-threo-methylphenidate, can be prepared in the following manner:
Composition (for 1000 tablets)d-threo-methylphenidate 10.0 gramslactose328.5 gramscorn starch 17.5 gramspolyethylene glycol 6000 5.0 gramstalc 25.0 gramsmagnesium stearate 4.0 gramsdemineralized water q.s.
[0035] The solid ingredients are first forced through a sieve of 0.6 mm mesh width. Then the d-threo-methylphenidate, lactose, talc, magnesium stearate and half of the starch are intimately mixed. The other half of the starch is suspended in 65 milliliters of water and this suspension is added to a boiling solution of the polyethylene glycol in 260 milliliters of water. The resulting paste is added to the pulverulent substances, and the whole is mixed and granulated, if necessary with the addition of water. The granulate is dried overnight at 35° C., forced through a sieve of 1.2 mm mesh width and compressed to form tablets of approximately 10 mm diameter which are co...
example 3
[0037] The sodium lauryl sulfate is sieved into the d-threo-methylphenidate through a sieve of 0.2 mm mesh width and the two components are intimately mixed for 10 minutes. The microcrystalline cellulose is then added through a sieve of 0.9 mm mesh width and the whole is again intimately mixed for 10 minutes. Finally, the magnesium stearate is added through a sieve of 0.8 mm width and, after mixing for a further 3 minutes, the mixture is introduced in portions of 28 milligrams each into size 0 (elongated) gelatin dry-fill capsules.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| weight loss | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com