Heart wall tension reduction apparatus and method
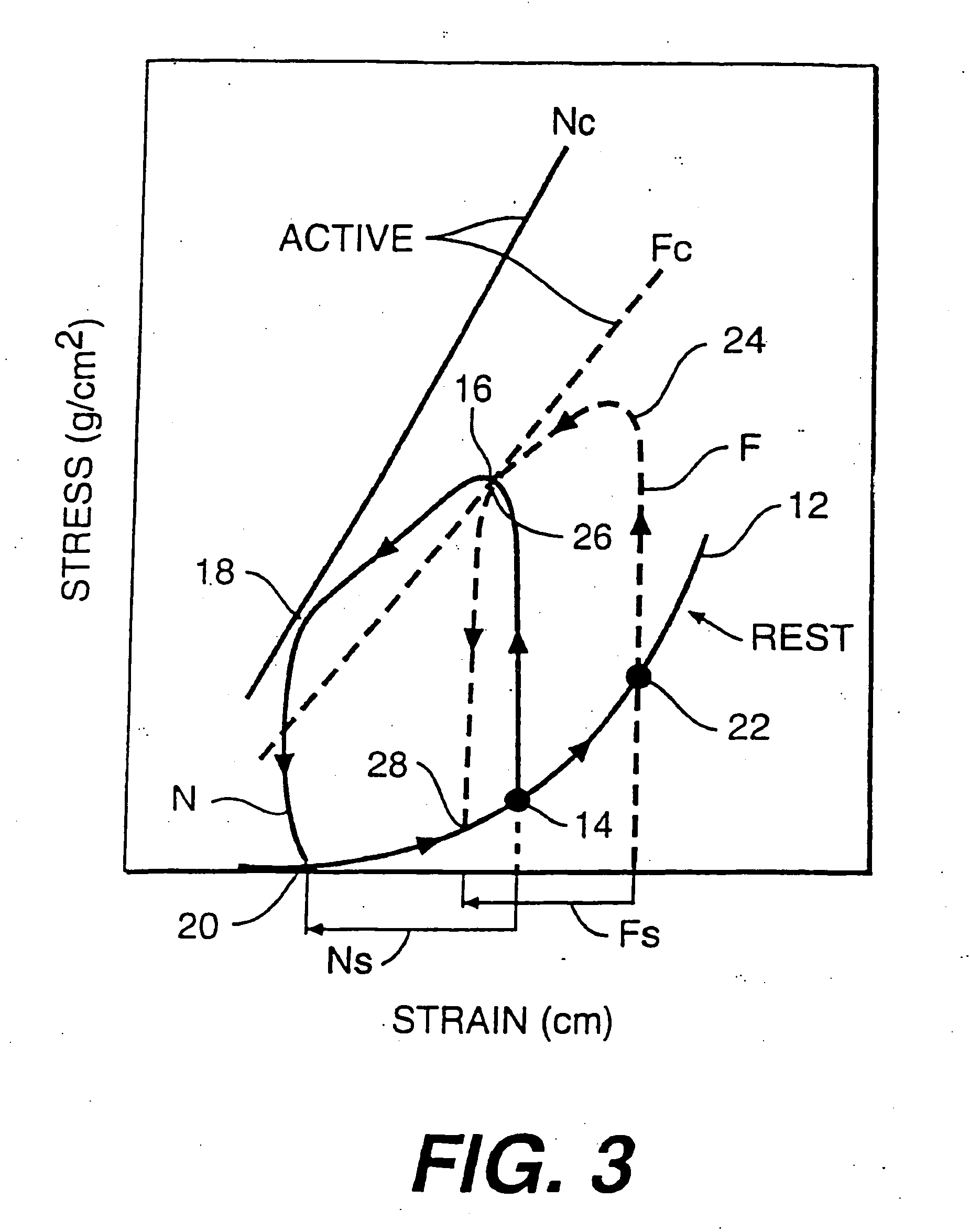
a technology for reducing the maximum wall tension and reducing the effect of wall tension
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
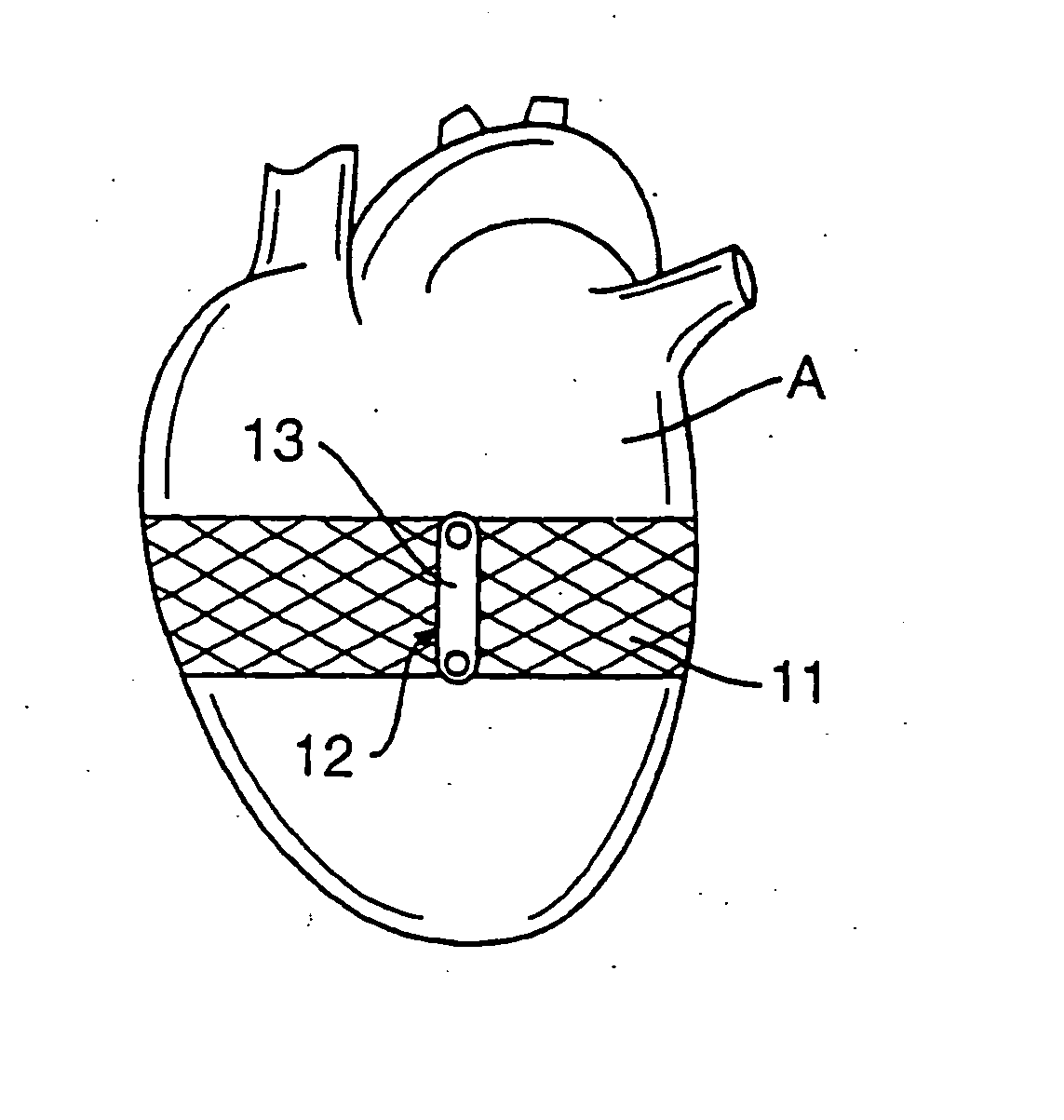
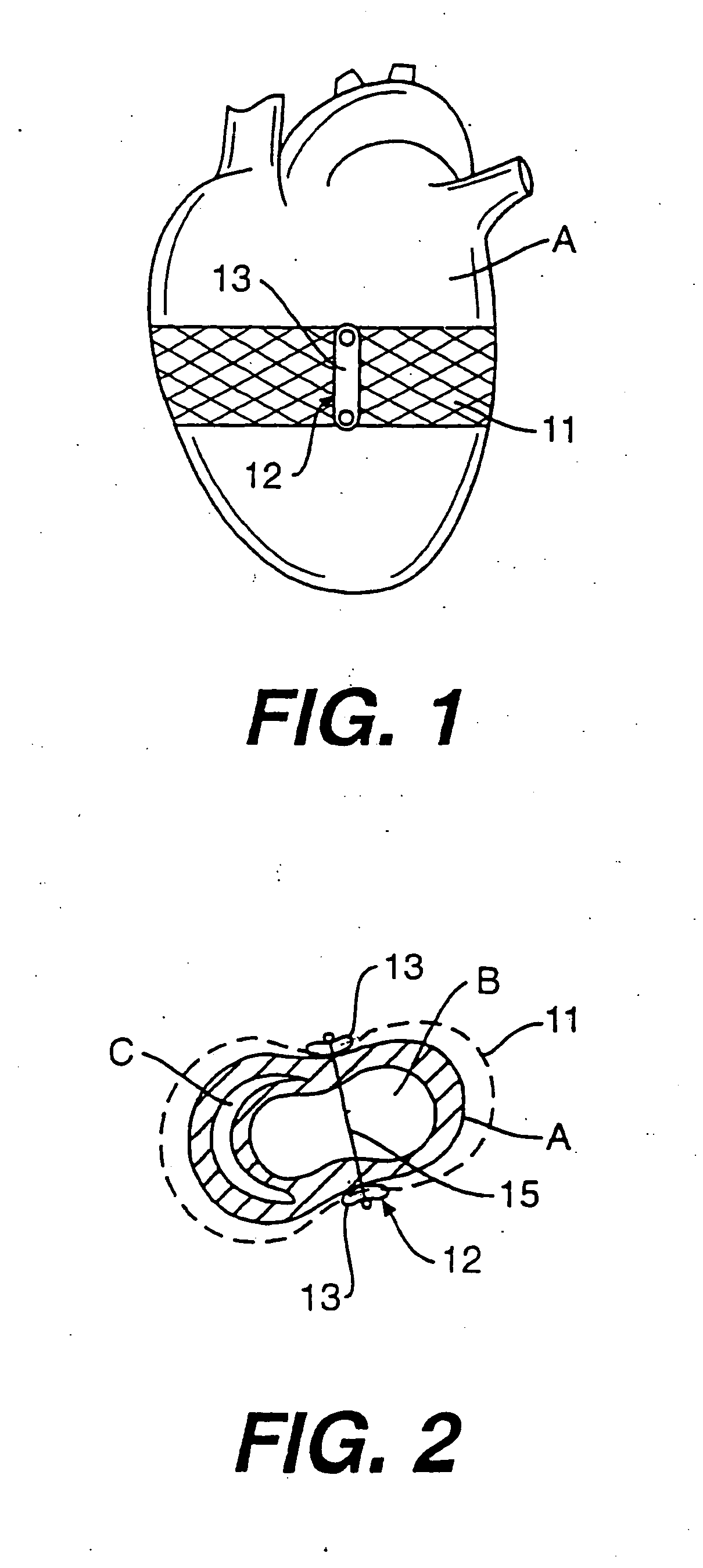
embodiment 110
[0088]FIG. 13 is an alternate embodiment 110 of the band splint of FIG. 12. Band splint 110 includes a horizontally heart encircling band 111 and four bands 113 extending downward from band 111. Bands 113, however, unlike bands 102 of band splint 100 do not extend to the apex of heart A, but rather to a second horizontally heart encircling band 112.
[0089] Band splint 110 could be made of the same materials as band splint 100. Band splint 110 can also be used in a manner similar to band splint 100 except that band splint 110 would limit the vertical elongation of the ventricles less than band splint 100.
[0090]FIG. 14 is yet another alternate embodiment 120 of the wrap of FIG. 12. Band splint 120 closely resembles alternate embodiment 110 of FIG. 13, except that rather than having four vertically extending web members, band splint 120 includes two substantially rigid members 123 interconnecting two horizontally encircling web members 121 and 122.
[0091]FIG. 15 is yet another alternat...
embodiment 170
[0096]FIG. 19 is an alternate embodiment 170 of the wrap of FIG. 18. Wrap 170, however, includes two vertically extending bars 172 having eyelets 173 through which line 171 is threaded. Line 171 can be tied to one of the bars 172 at 174 and 175.
[0097]FIG. 20 is a vertical view of heart A including yet another embodiment 180 of the wrap of FIG. 18. Wrap 180 includes a line 181 encircling heart A a plurality of times. Rather than having a single vertically extending bar 162 to position line 180 on heart A, wrap 180 includes a plurality of horizontal bars 182 including a pair of eyelets 183. One end of line 181 is tied to an upper bar 182 at 184 and the opposite end of line 181 is tied to a lower bar 182 at 185. Between 184 and 185, line 181 is threaded through eyelets 182 to form the heart encircling pattern shown in FIG. 20.
[0098]FIG. 21 is a vertical view of heart A including yet another alternate embodiment 190 of the wrap of FIG. 18. Wrap 190 closely resembles 180 of FIG. 20. Lin...
embodiment 270
[0108]FIG. 31 is a horizontal cross sectional view of heart A including left ventricle B and right ventricle C and an alternate embodiment 270 of the device of FIG. 30. Device 270 includes a line 271 which does not extend transventricularly but extends through the myocardium of heart A to form four loops 273.
[0109] Device 270 can be formed from material similar to that used to form device 260. Additionally, device 270 can be made to function as a restrictive device or full cycle device in a manner similar to that of device 260.
[0110] Line 261 and line 267 could be disposed within a tube to avoid cheese cutting-of the myocardium. The tube may be highly flexible, yet durable enough to prevent the line from cheese cutting through the myocardium of the heart. Devices 260 and 270 could extend through the septum or right ventricle to avoid forming lobes in right ventricle C.
[0111]FIG. 32 is a vertical view of heart A including three devices 270 disposed at three spaced elevations. An el...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com