Method and apparatus for chemical synthesis
a chemical synthesis and apparatus technology, applied in the field of large scale, can solve the problems of limited yield, large quantity of monosilane, low yield of higher-order silanes, etc., and achieve the effect of facilitating continuous operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
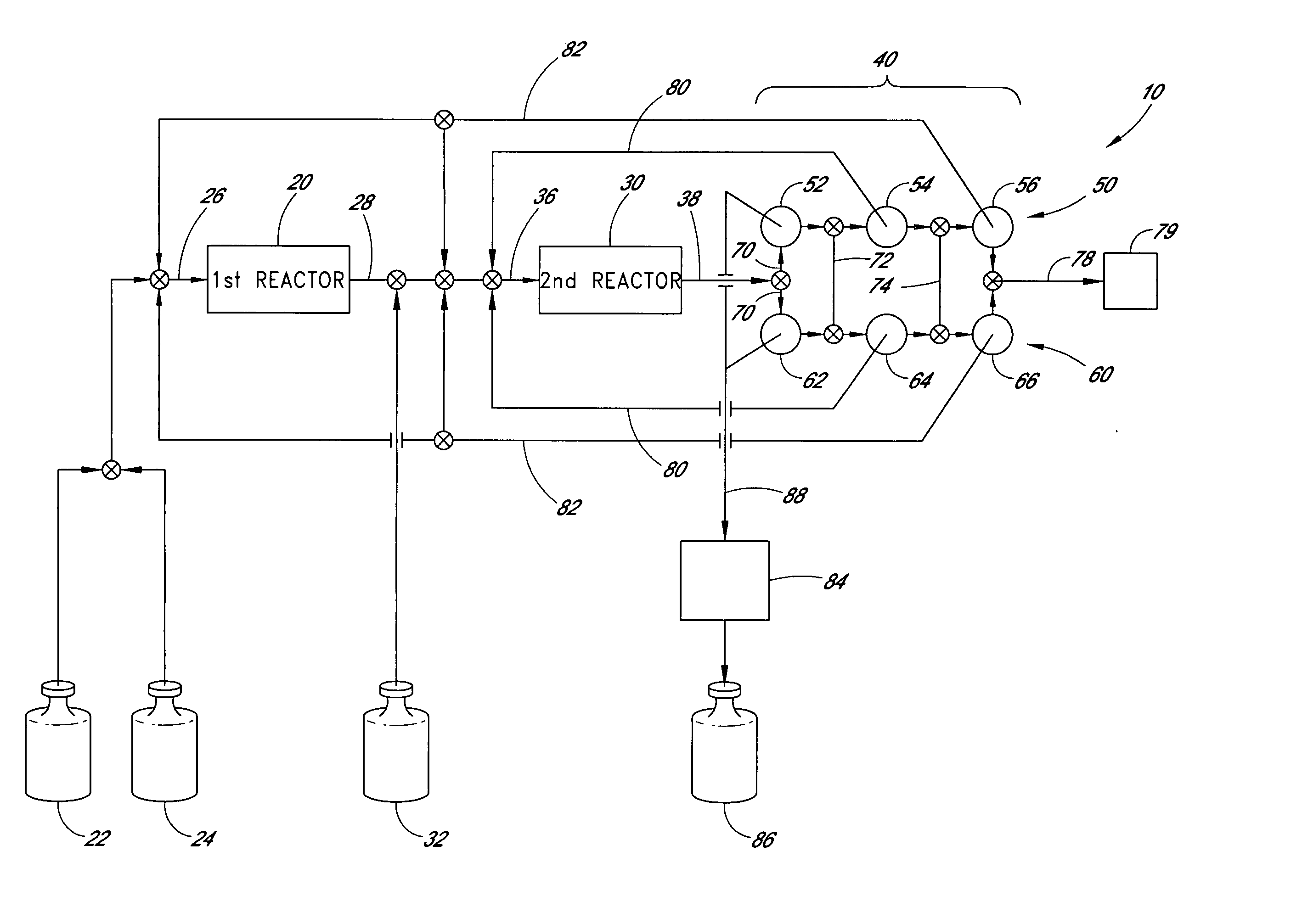
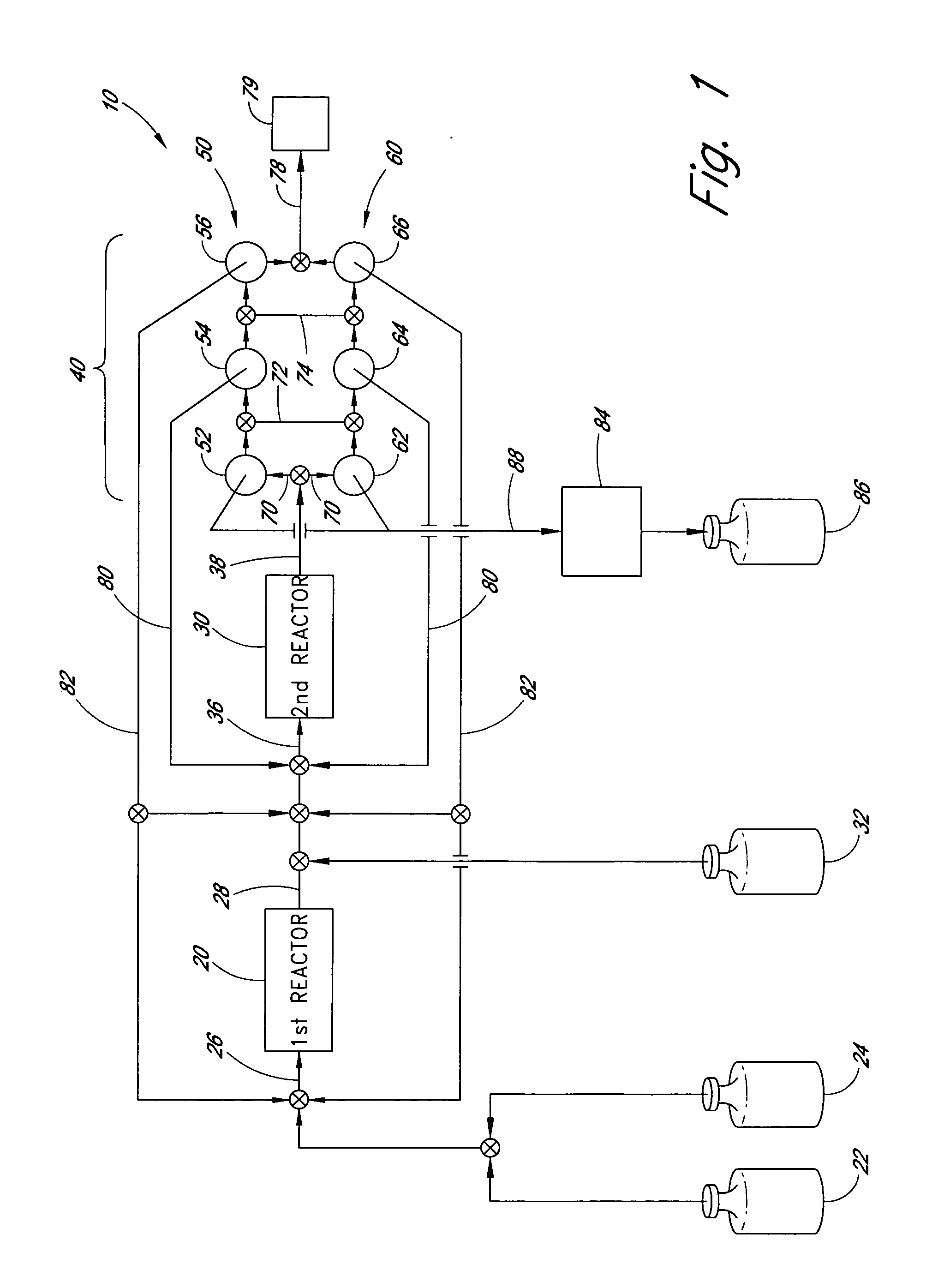
[0054] An exemplary apparatus and process is provided below for the production of trisilane from monosilane.
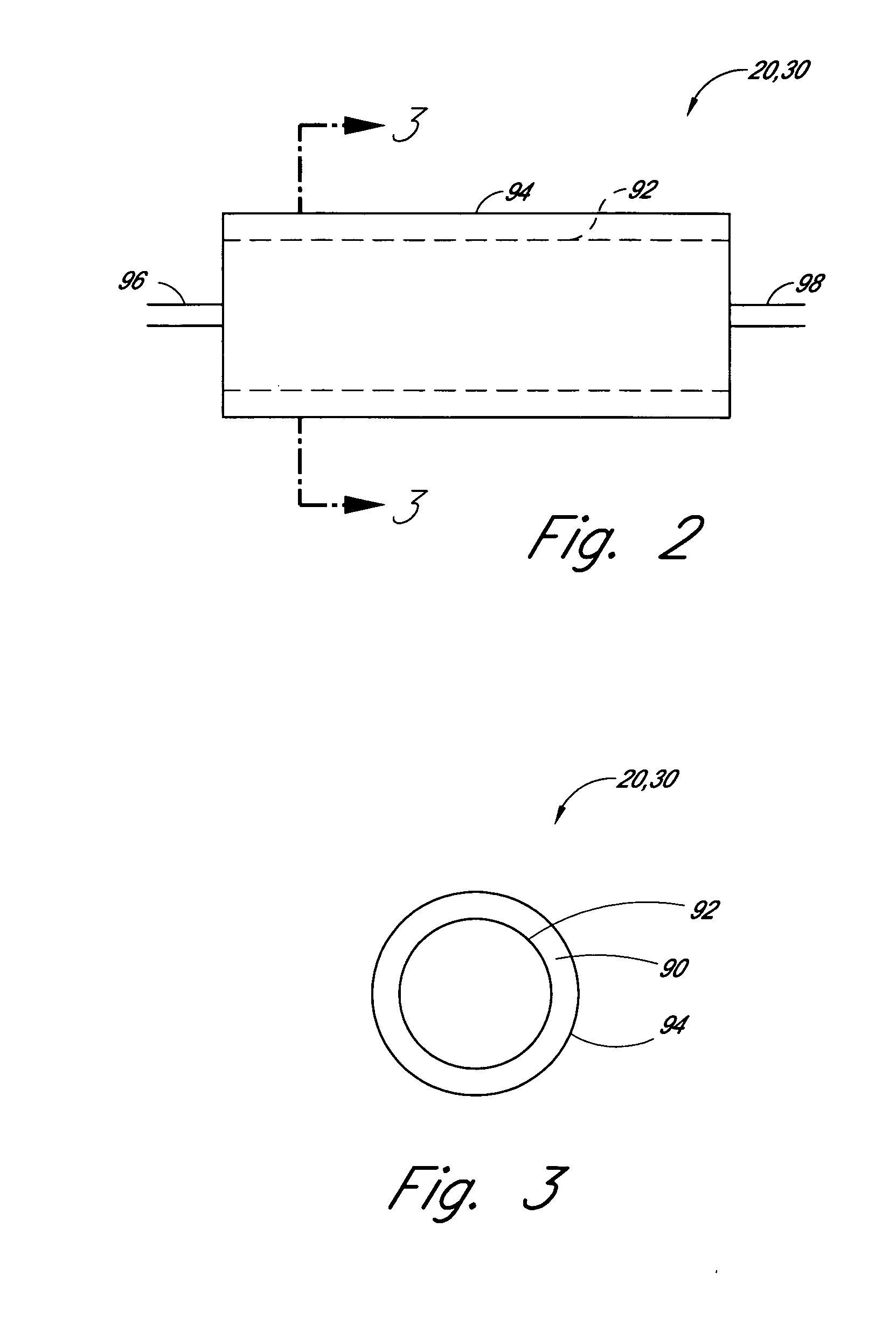
[0055] First Reactor: A 5 cm long concentric stainless steel tube with inner and outer walls separated by evacuated space, inner wall is grounded, serving as the outer electrode for the reactor. The evacuated space between the inner and outer stainless steel walls serves as thermal insulation. Within the inner stainless steel wall is another cylindrical element comprising alumina (or other suitable dielectric material) coated with a low work function metal (e.g., silver, tungsten, etc. . . . ), connected to high frequency electric source via contact and conductor, also 5 cm in length. The inner stainless steel wall and the concentric metal-coated alumina cylinder (i.e., the electrodes) are spaced about 0.1 cm apart, defining the reaction space therebetween. A conduit is provided for feeding reactants to the volume present between the two electrodes and a conduit is provided f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com