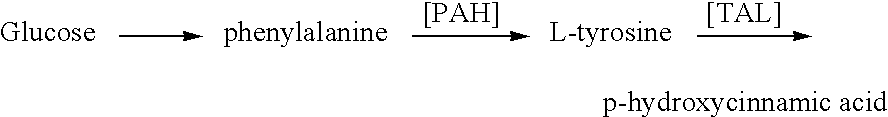Methods for the production of tyrosine, cinnamic acid and para-hydroxycinnamics acid
a technology of cinnamic acid and tyrosine, which is applied in the field of molecular biology and microbiology, can solve the problems of time-consuming and cumbersome methods, and low supply of tyrosine, and achieves enhanced tal activity and enhanced tal activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Cloning and Expression of Chromobacterium violaceum Phenylalanine Hydroxylase in Microorganisms
Phenylalanine Hydroxylase for Chromobacterium violaceum DNA Amplification and Cloning:
[0124] In these studies the PAH gene of the Chromobacterium violaceum (SEQ ID NO:1) was cloned into both Pseudomonas aeruginosa (ATCC 15691) and DH5αE. coli. Two oligonucleotide primers, 5′-TCCAGGAGCCCAGGATCCAACGATCGCGCCGA-3′ [SEQ ID NO:5], (designated CVPH168), and 5′-GGACAAGCTTAATGATGCAGCGACACAT-3′ [SEQ ID NO:6], (designated CVPH1170) were synthesized based on the deoxynucleotide sequences flanking the coding region of the C. violaceum phenylalanine hydroxylase gene described previously (A. Onishi, L. J. Liotta, S. J. Benkovic; Cloning and Expression of Chromobacterium violaceum phenylalanine hydroxylase in Escherichia coli and comparison of amino acid sequence with mammalian aromatic amino acid hydroylase J. Biol. Chem. 266: 18454-18459 (1991)). Restriction endonuclease sites (BamHI or HindIII, unde...
example 11
Growth of E. coli AT271 Tyr-Auxotrophic Strain Following its Transformation with C. violaceum PAH and the Various Components of the P. aeruginosa PAH Operon
[0127] Agar plates containing M9 medium plus glucose were prepared. The tyrosine auxotrophic AT271 strain is incapable of growing on this plate in the absence of tyrosine (see Control-1). When a filter disc containing 1.0 mM tyrosine is added to the plate, growth is observed around the disc indicating the dependency of the organism to the presence of tyrosine (see Control-2). The AT271 recombinant strains used in this study included strains containing PhhA, PhhB, PhhC, PhhBC, PhhABC, PAH and PAH / PhhB / PhhC. The tyrosine disc was placed in the middle of each of the plates for these recombinants. For the strains containing PhhA, PhhB, PhhC, and PhhBC growth appeard only around the tyrosine disc indicating that these strains could not synthesize tyrosine and were still dependent on the external supply of this compound for growth. Ho...
example 12
The Effect of Iron on Growth and Tyrosine Production by Transformants of Phenylalanine Overproducing E. coli Containing the PAH Gene
[0128] In order to examine the effect of iron on the activity of PAH and therefore production of tyrosine, E. coli strains containing the PAH gene of the C. violaceum (PAH) [SEQ ID NO:1] and the PhhA component of the Pseudomonas PAH operon were grown in the M9 medium with glucose with and without addition of either FeSO4 or Fe(NH4)2(SO4)2 (1.0 M final conc.). Samples were taken at 2.0, 6.0, 16.0, and 22 hours. The results are shown in the Table 13. Based on the results obtained, it was concluded that addition of neither of the two iron sources had any significant positive effect on the level of the tyrosine produced.
TABLE 13Tyrosine Production of Phenylalanine OverproducingStrain (ATCC31884) Transformed with PAHfrom C. violaceum in the Presence and Absence of Fe+2Time (hour)Presence of Fe+2Absence of Fe+20006132.68146.7616173.79171.7722212.99208.58
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com