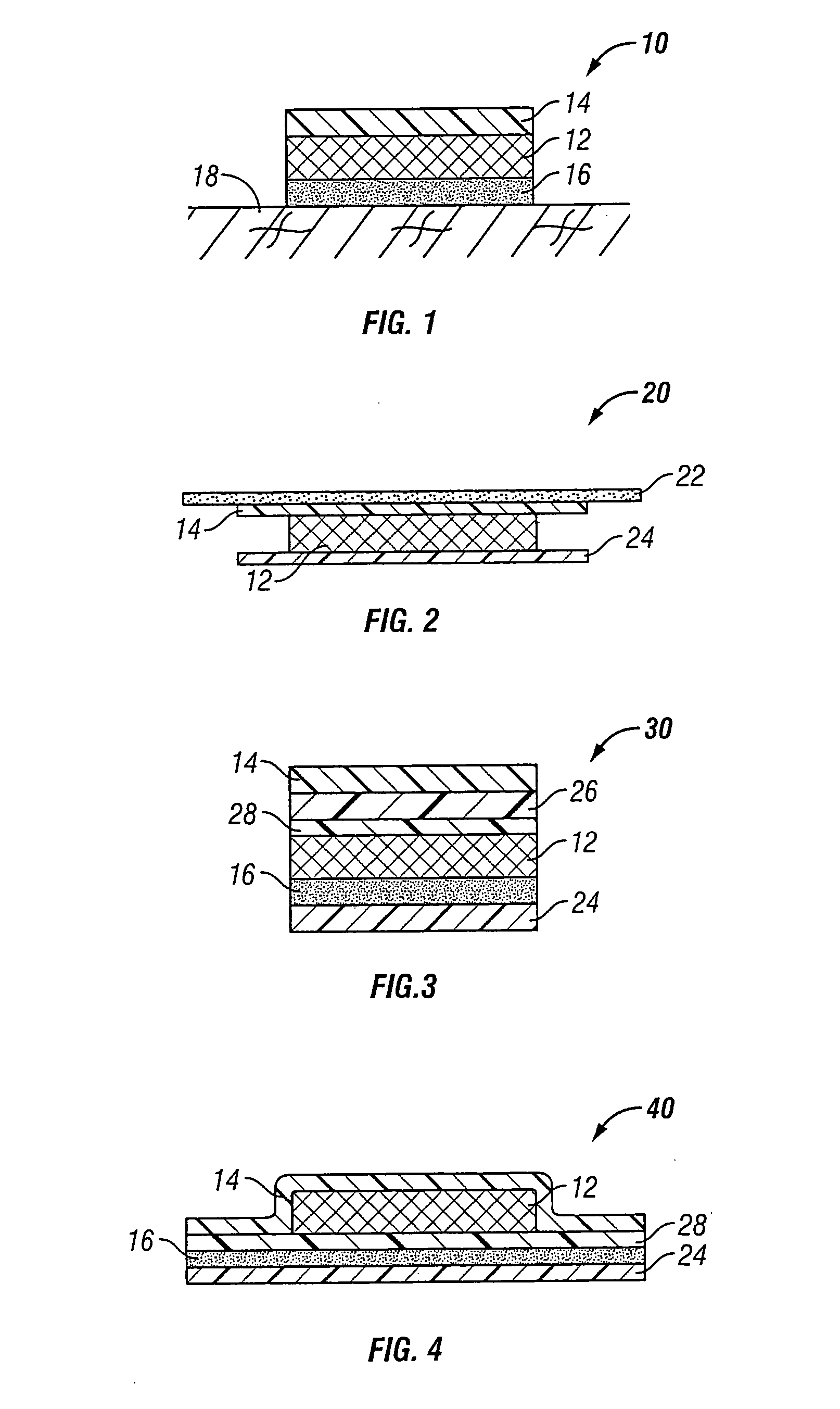
Novel formulations for the administration of fluoxetine
a formulation and fluoxetine technology, applied in the field of sustained release formulations, can solve the problems of significant delay in the onset of therapeutic effect, the assumption of potential, and the inability to meet the initial expectations of the transdermal route, so as to achieve safe and efficacious administration and reduce skin irritation levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
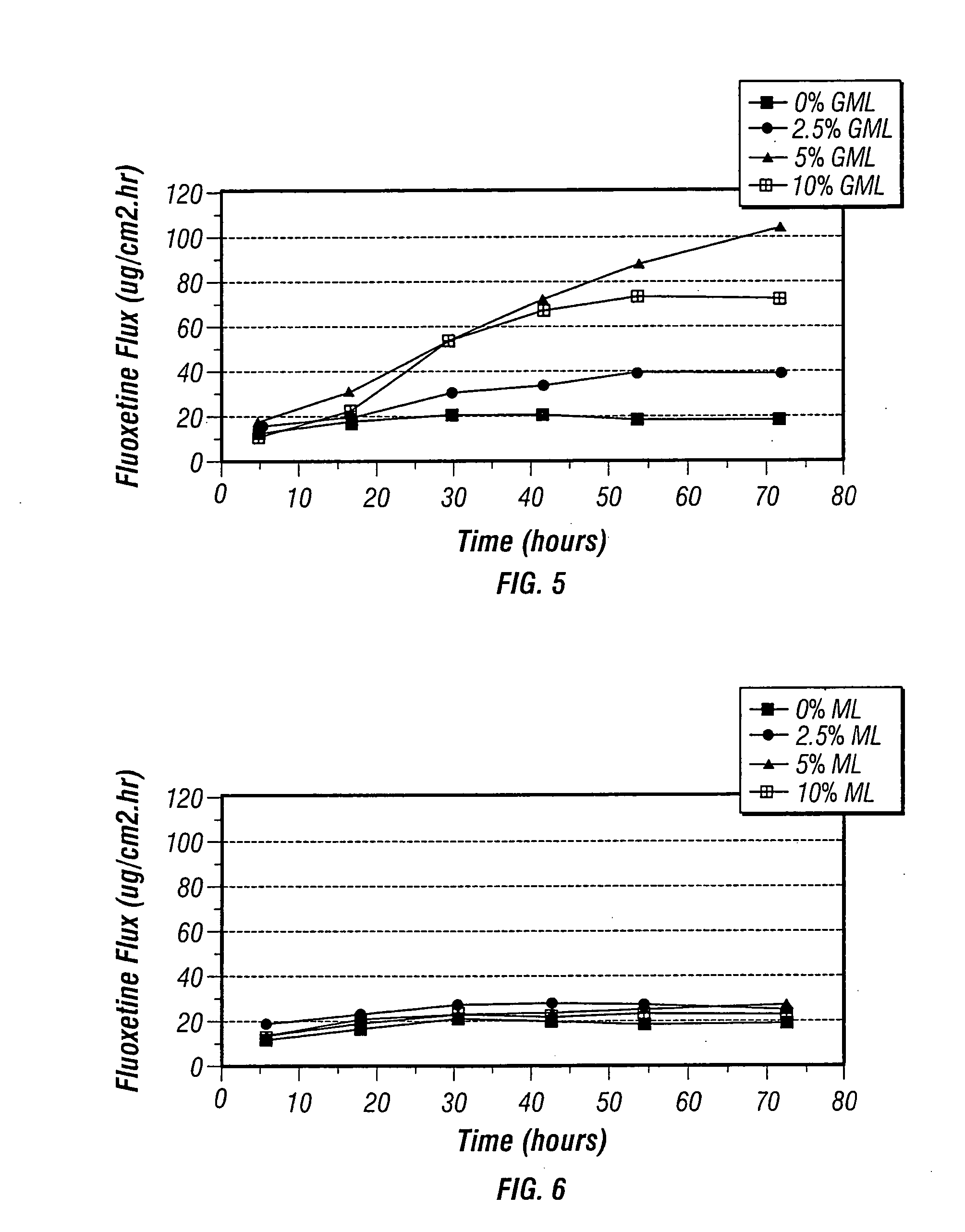
[0143] Several test samples were made to measure the flux of fluoxetine base through human cadaver epidermis from donor vehicles containing 10% by weight fluoxetine base in an oil / petrolatum carrier with 0- 10% by weight glycerol monolaurate (GML) as shown in Table 1.
TABLE 1Non-Aqueous Donor Solutions (weight percent)Symbol(FIG. 5)FluoxetineGMLOil / Petrolatum▪10090●102.587.5▴10585101080
[0144] The experiment was carried out using standard glass diffusion cells which consist of a donor compartment with a 7.5 ml capacity, and a receptor compartment with a 22 ml capacity. A circular piece of epidermis was placed in each diffusion cell (permeation area=2.27 cm2) in a horizontal position between a lower capped receptor compartment and an upper capped donor compartment. The receptor compartment has both a venting tube (uncapped) and a sampling port (capped). The stratum corneum side of the epidermis faced the donor compartment. An O-ring was positioned between the epidermis and the donor ...
example 2
[0147] Several test samples were made to measure the flux of fluoxetine base through human cadaver epidermis from donor vehicles containing 10% by weight fluoxetine base in an oil / petrolatum carrier with 0-10% by weight methyl laurate (ML) as shown in Table 2. Transdermal fluxes were obtained using human epidermis at 35° C in standard diffusion cells using the procedure set forth in Example 1. FIG. 6 graphically depicts the results. As seen in FIG. 6, methyl laurate by itself did not significantly increase the fluoxetine flux.
TABLE 2Non-Aqueous Donor Solutions (weight percent)Symbol(FIG. 6)FluoxetineMLOil / Petrolatum▪10090●102.587.5▴10585101080
example 3
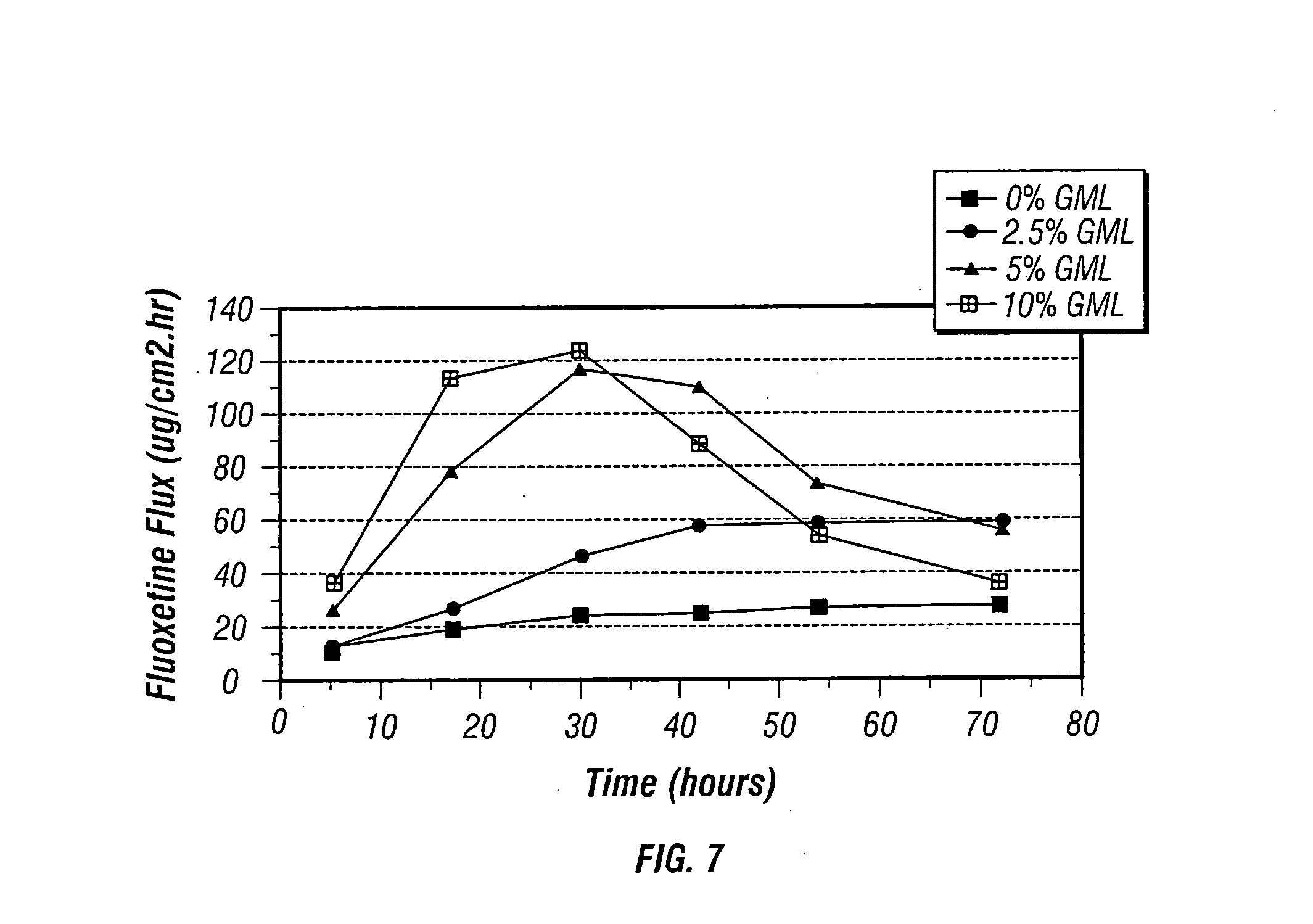
[0148] Several test samples were made to measure the flux of fluoxetine base through human cadaver epidermis from donor vehicles containing 10% by weight fluoxetine base in an oil / petrolatum carrier with 5% by weight methyl laurate (ML) and 0-10% by weight GML as shown in Table 3. Transdermal fluxes were obtained using human epidermis at 35° C. in standard diffusion cells using the procedure set forth in Example 1. As seen in FIG. 7, the combination of methyl laurate and GML exhibited a synergistic effect on the flux of fluoxetine as compared to the enhancement of flux in the presence of GML or methyl laurate individually.
TABLE 3Non-Aqueous Donor Solutions (weight percent)Symbol(FIG. 7)FluoxetineGMLMLOil / Petrolatum▪100585●102.5582.5▴1055801010575
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface area | aaaaa | aaaaa |
| weight % | aaaaa | aaaaa |
| area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com