Electroplating apparatus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
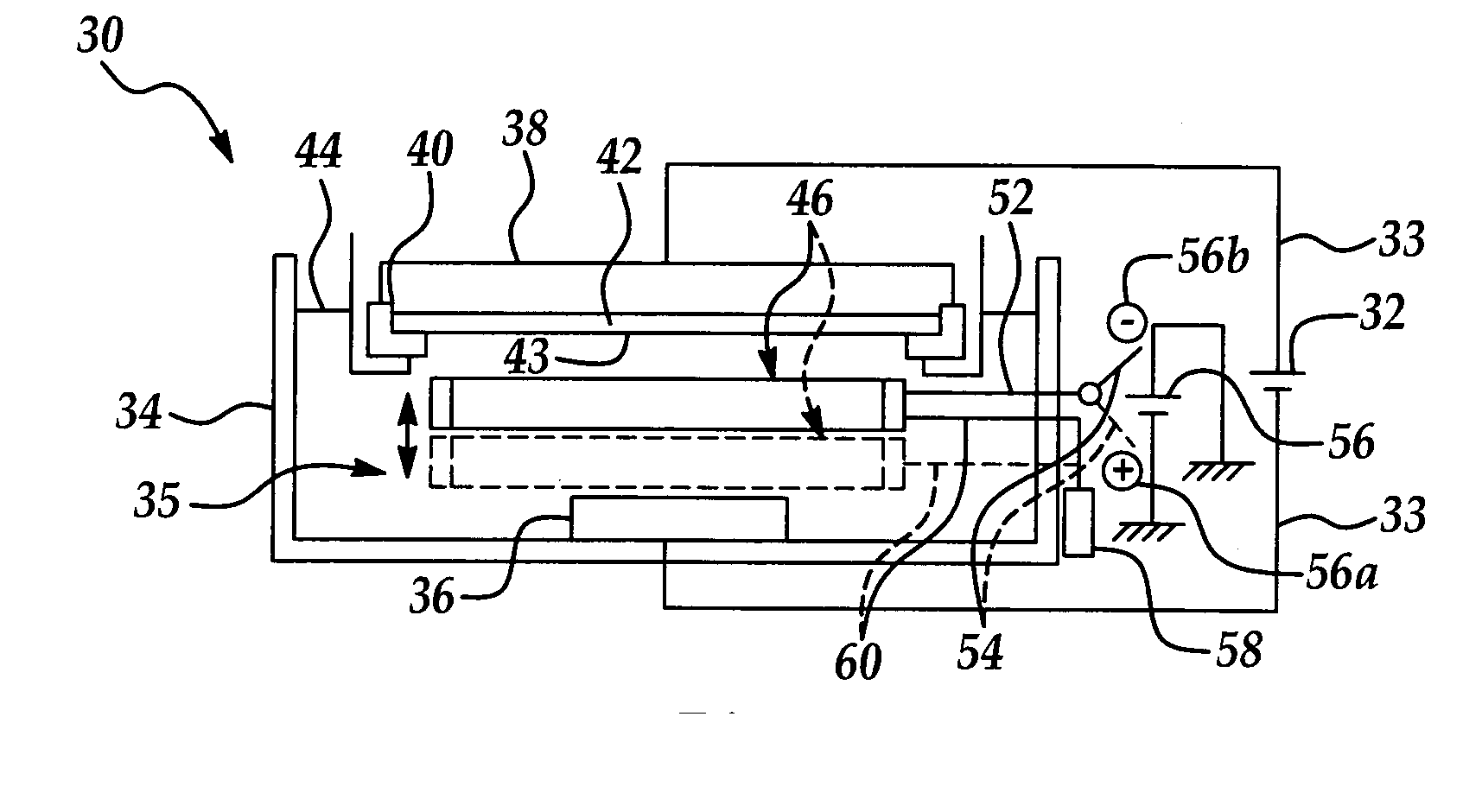
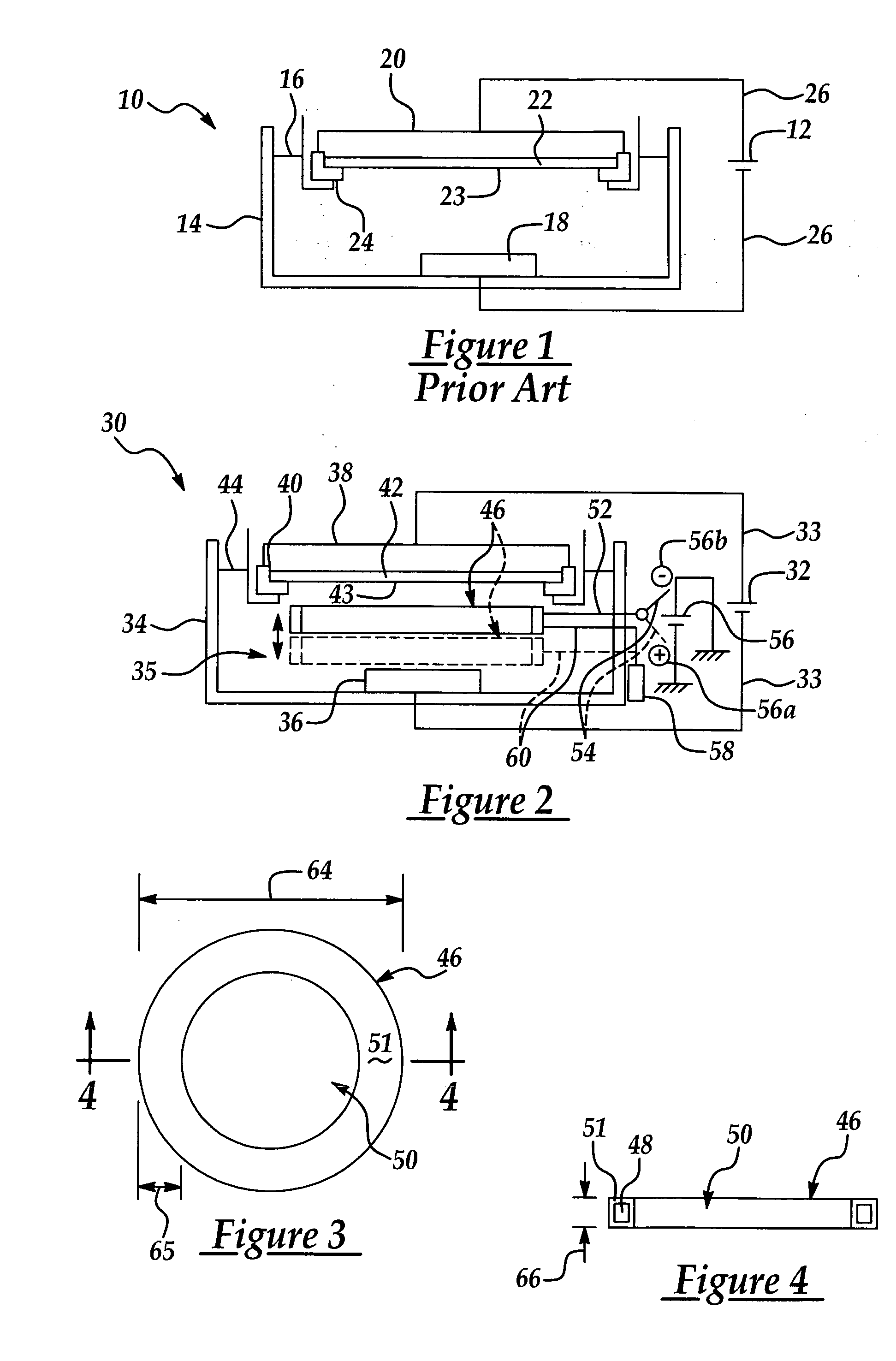
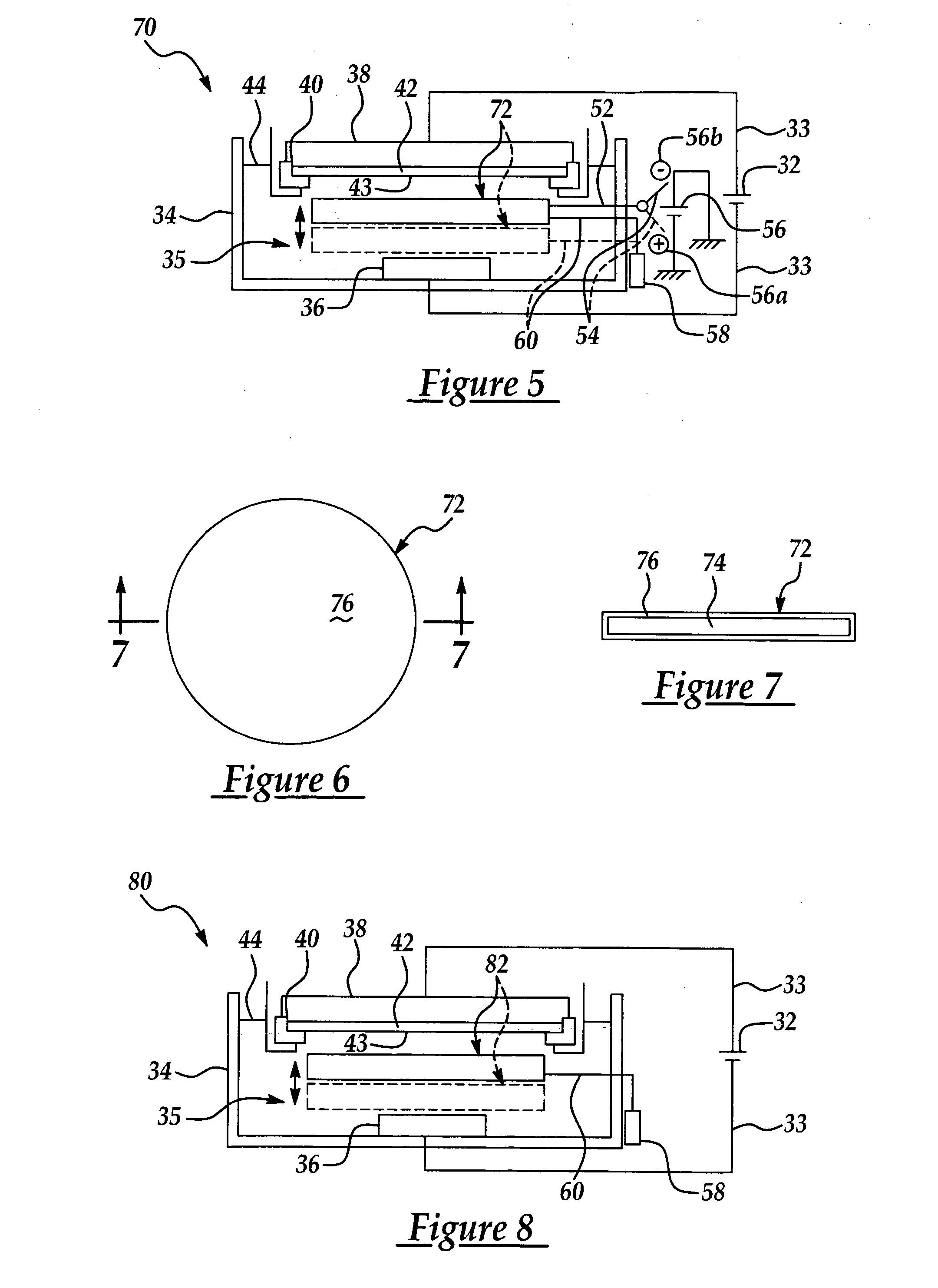
[0034] The present invention has particularly beneficial utility in the electrochemical plating of copper or other metal onto a semiconductor wafer substrate in the fabrication of semiconductor integrated circuits. However, the invention is more generally applicable to the electrochemical plating of metals including but not limited to copper on substrates in a variety of industrial applications, including but not limited to semiconductor fabrication.
[0035] The present invention is generally directed to a novel electroplating apparatus which enhances uniformity in the thickness of a metal layer deposited on a semiconductor wafer. The apparatus facilitates the electroplating of a metal layer having substantially uniform thickness across the entire wafer surface, particularly between the center and edge regions of the wafer. The apparatus includes a bath container having a reservoir for containing an electrolytic fluid. A cathode and an anode immersed in the electrolytic fluid are con...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Electric charge | aaaaa | aaaaa |
| Current | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com