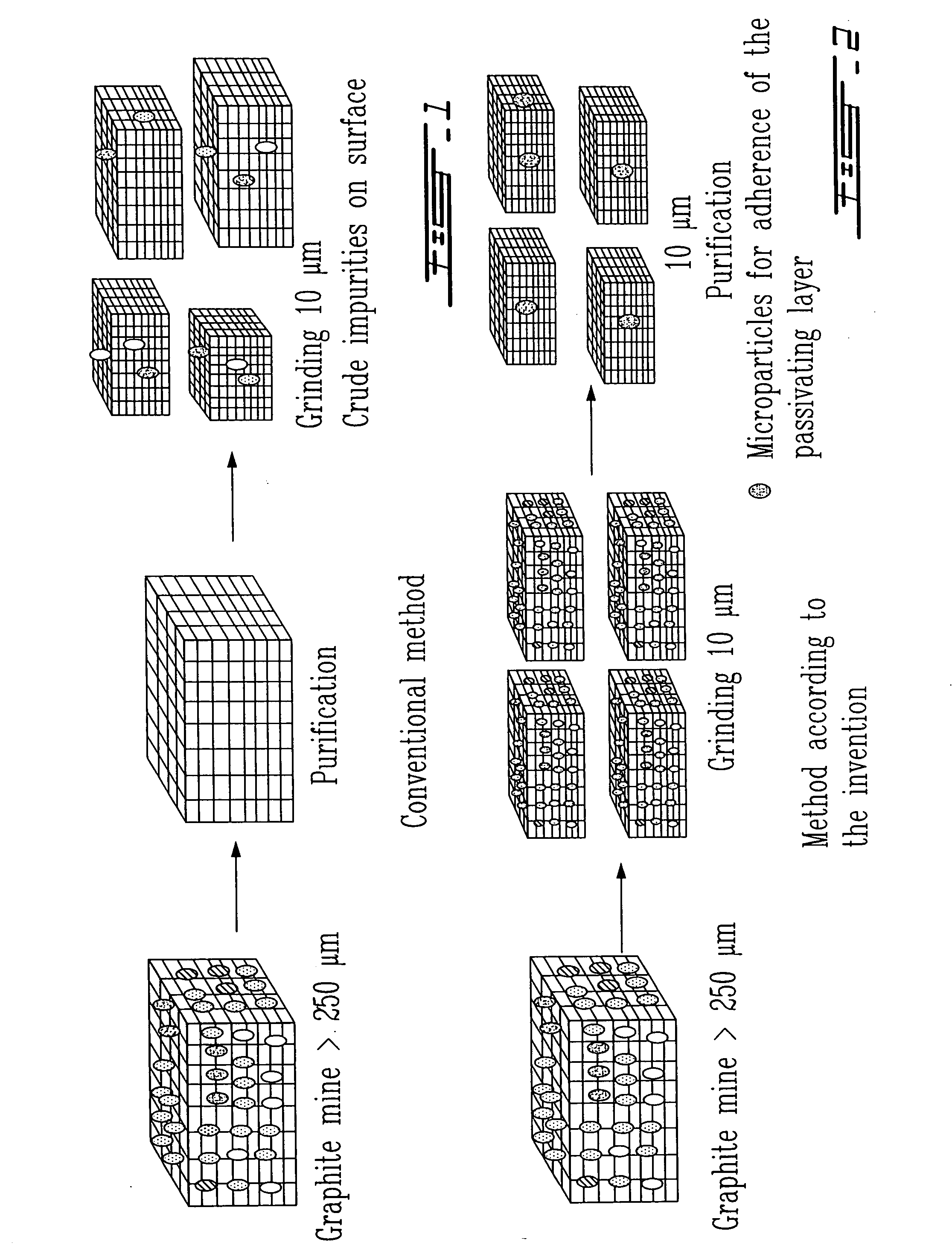
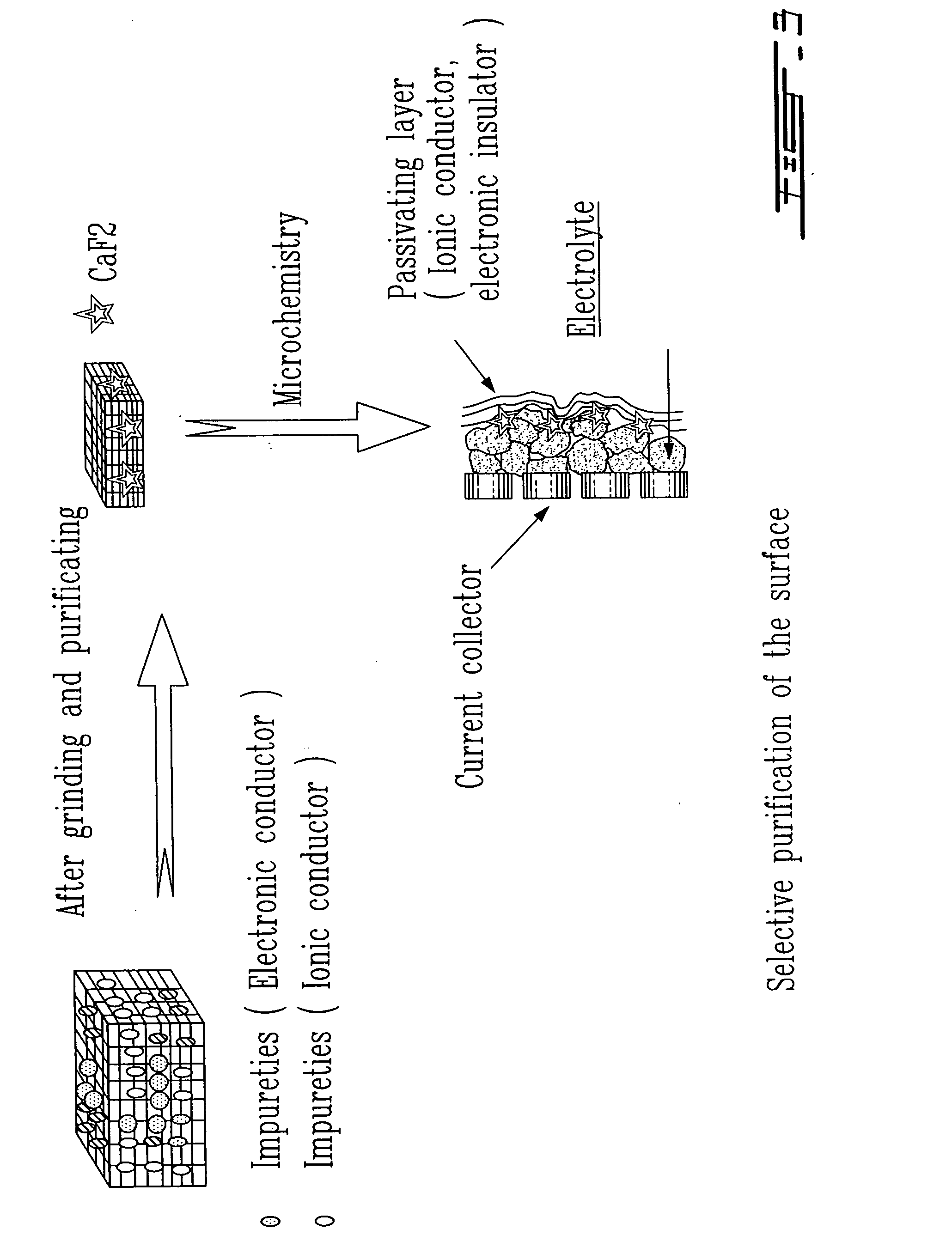
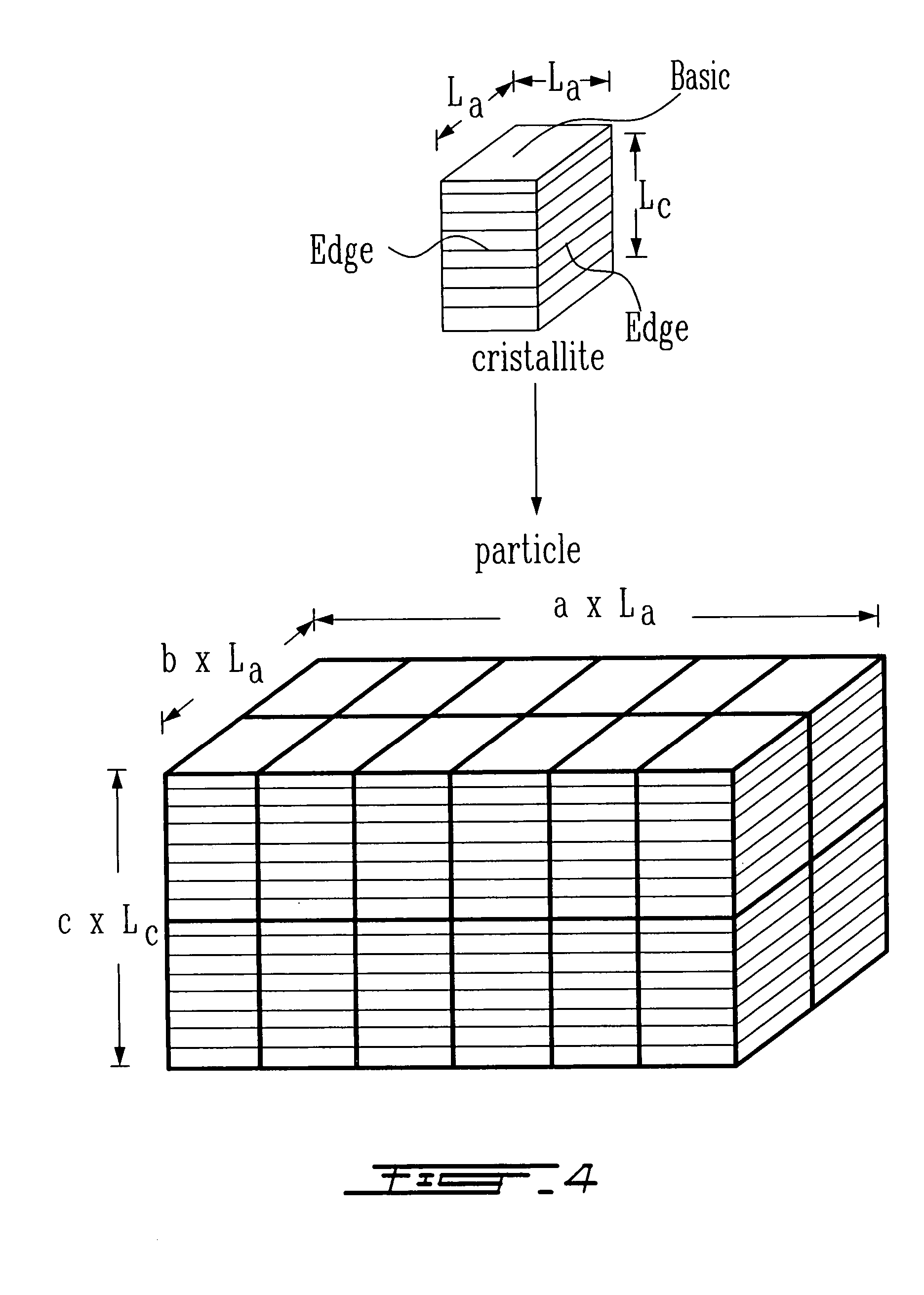
Surface preparation of natural graphite and the effect of impurities on grinding and the particle distribution
a technology of natural graphite and impurities, which is applied in the field of surface preparation of natural graphite and the effect of impurities on grinding and particle distribution, can solve the problems of reducing the mobility and maximum concentration of lithium batteries inside the layers, and slowing the arrival of lithium batteries in the classical formats (aa, c, d, etc.) to the public at larg
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0049] A natural graphite having an initial particle size of 375 μm is ground by a process of air milling until the particle size reaches 10 μm. The size of the main particles obtained (50% distribution of particles or D50%) is 10.52 μm. The Gaussian distribution of graphite has only one maximum and no additional peak. The granulometric distribution was determined with the aide of a Microtrac™ particle analyser built and sold by Leeds & Northrul. The methanol was used as the carrier fluid. Subsequently, the ground graphite was leached in an aqueous bath of 30% HF. The temperature of the mixture is maintained at 90° C., with a leached time of 180 minutes. The graphite is then filtered, washed with copious amounts of water, and the powder dried for a period of 24 hours at 120° C.
[0050] The graphite powder obtained is analysed by reversed diffusion coupled with EDX (Energy Dispersive X-ray). No exfoliation of the particles was observed. In addition the analysis by EDX shows that the m...
example 2
[0054] Natural graphite having an initial particle size of 375 μm is ground by a process of air-milling until the particles attain a size of 10 μm. The graphite is then leached in a mixed aqueous bath comprising 30% H2SO4 and 30% HF. 106.5 ml of the mixed acid is heated to 90° C., and 30 g of graphite is then added into the solution. The graphite is leached for 180 minutes in a reactor. The solid is then filtered, washed with copious amounts of water, and dried at 120° C. for 24 hours. The size (D50%) is 10.92 μm, and this before and after purification. The Gaussian distribution for the graphite has only one single maximum without any peak.
[0055] The analysis of impurities in the graphite by EDX shows mainly the presence of the elements Ca and F. Analysis of the impurities of the residual ashes shows the graphite purity to be 99.68%. The electrode preparation and the electrochemical tests are done using the same procedure described in example 1.
[0056] The coulombic efficiency of t...
example 3
[0057] The natural graphite used in this example is processed in an identical manner as that described in Example 2, with the exception that the HF concentration is now 20%. Analysis of the impurities in the graphite by EDX shows the main presence of the elements Ca and F. The analysis of the impurities of the residual ashes shows the graphite to have a purity of 99.75%. The preparation of the electrode and the electrochemical tests were conducted with the identical procedures described in example 1.
[0058] The coulombic efficiency of the first cycle is 89%. The irreversible plateau due to the passivating layer is formed normally near 800 mV. The reversible capacity of the graphite is 365 mAh / g, equivalent to x=0.98 for the formation LixC6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com