Therapeutic agent delivery apparatus with direct mechanical ventricular assistance capability
a technology of mechanical ventricular assistance and therapeutic agent, which is applied in the field of therapeutic agent delivery apparatus with direct mechanical ventricular assistance capability, can solve the problems of loss of life before adequate circulatory support, non-blood contacting devices similar, and insufficient cardiac output, so as to achieve the effect of simple procedure and quick performan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0130] For a general understanding of the present invention, reference is made to the drawings. In the drawings, like reference numerals have been used throughout to designate identical elements.
[0131] In describing the present invention, a variety of terms are used in the description. Standard terminology is widely used in cardiac art. For example, one may refer to Bronzino, J. D., The Biomedical Engineering Handbook, Second Edition, Volume I, CRC Press, 2000, pp. 3-14 and 418-458; or Essential Cardiology, Clive Rosendorf M.D., ed., W.B. Saunders Co., 2001, pp. 23-699, the disclosures of which are incorporated herein by reference.
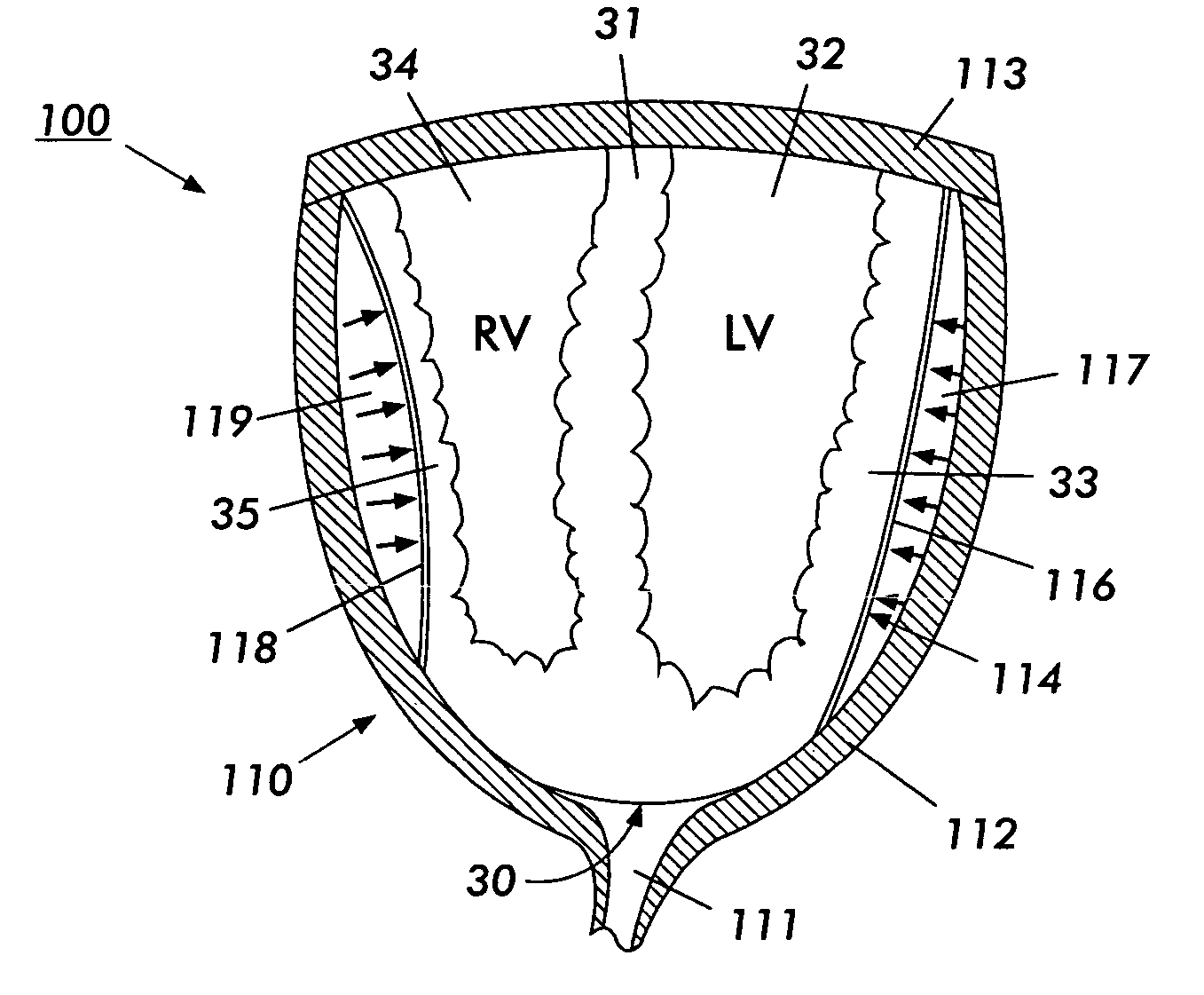
[0132] As used herein, the term Cup is meant to indicate the Direct Mechanical Ventricular Assist device of the present invention, such device comprising a cup-shaped outer shell. The terms Cup, DMVA Cup, DMVA device, and DMVA apparatus are used interchangeably in this specification and are intended to denote the overall Direct Mechanical Ventricular Ass...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com