Process for preparation of mycophenolate mofetil and other esters of mycophenolic acid
a technology of mycophenolic acid and mofetil, which is applied in the field of process for the preparation of mycophenolate mofetil and other esters of mycophenolic acid, can solve the problems of high incidence of side effects and withdrawal of mpa
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
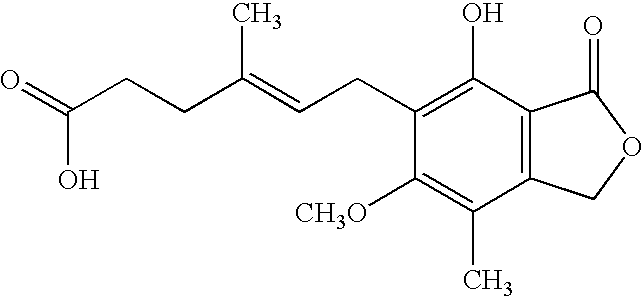
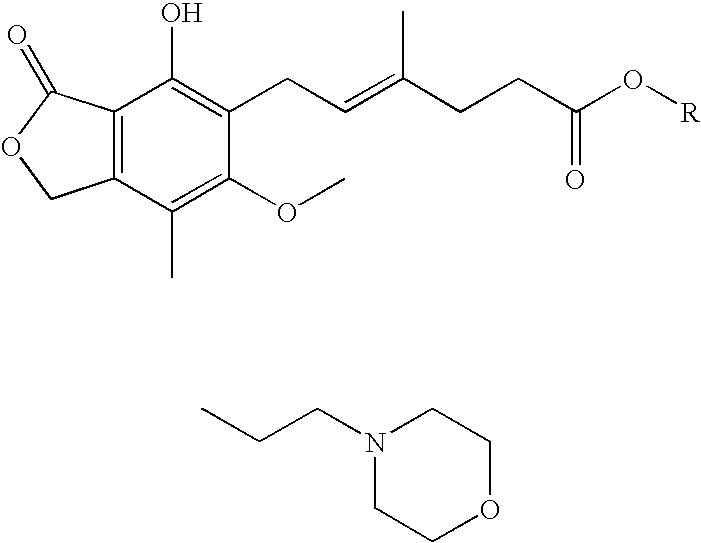
[0082] A mixture of mycophenolic acid (192 g, 0.6 mol) and 4-(2-hydroxyethyl)morpholine (440 ml, 6 molar equivalents) was stirred at 150-155° C. for 4 hours in the presence of tin(II) chloride dihydrate (20.4 g, 0.15 molar equivalents) under nitrogen atmosphere. After the completion of the reaction, the reaction mixture was allowed to cool to room temperature. The obtained dark liquid was poured into isobutyl acetate (4.0 l). The solution was extracted with 2% of aqueous sodium bicarbonate solution (1.2 l, then 2×0.4 l). After the first addition of sodium bicarbonate solution, the formed two-phase system was treated with charcoal (40 g) and filtrated (an emulsion was filtered off). The solution was extracted with water (1 liter). After phase separation the organic phase was washed with water (1 liter) and evaporated to dryness at 40-50° C. under vacuum. To the solid material acetone (400 ml) and isopropanol (3.8 l) were added and the mixture was warmed to 40-45° C. The material was ...
example 2
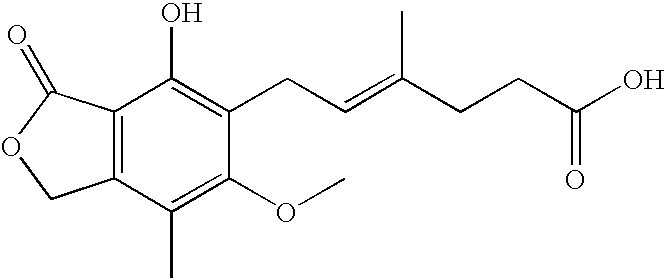
[0084] A mixture of mycophenolic acid (9.60 g, 30 mmol), 4-(2-hydroxyethyl)morpholine (14.7 ml, 4 molar equivalents) and (+)-camphorsulfonic acid (0.21 g, 0.9 mmol, 3 mol %) was stirred at 150-155° C. for 8 hours. After cooling to room temperature, water (200 ml) was added to the reaction mixture, and the mixture was seeded and stirred for 2 hours. The solid material was filtered off, washed with water (100 ml) and dried at room temperature. The product was 10.93 g (84% yield).
[0085] MPA level: 2.4 area %.
example 3
[0086] A mixture of mycophenolic acid (64.07 g, 0.2 mol), 4-(2-hydroxyethyl)morpholine (98 ml, 4 molar equivalents) and potassium dihydrogenphosphate (0.82 g, 3 mol %) was stirred under nitrogen atmosphere at 165° C. for 3 hours. The cooled mixture was dissolved in toluene (700 ml) at room temperature, and the solution was washed with 5% aqueous sodium bicarbonate solution (2×700 ml). The organic phase after drying on sodium sulfate was decolorized with charcoal (30 g). To the stirred solution, n-heptane (1000 ml) was added and the mixture was warmed to 60° C. The solution was cooled to −10° C., and after 1 hour the crystals were filtered off and dried at room temperature. The crude product was 54.0 g (62% yield).
[0087] MPA level: 0.06 area %. Assay: 95.6%
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| reaction time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com