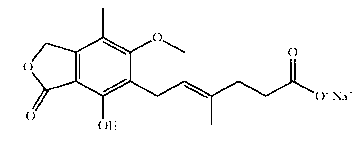
Matrix-type preparation containing mycophenolic acid or mycophenolic acid salt and coated tablet thereof
A technology of mycophenolate mofetil and mycophenolic acid, which is applied in the direction of medical preparations containing active ingredients, organic active ingredients, drug delivery, etc., can solve the problem of increasing the temperature of the substance and the increase in coating weight. Requirements, flammable and explosive problems, to achieve the effect of preventing precipitation and improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
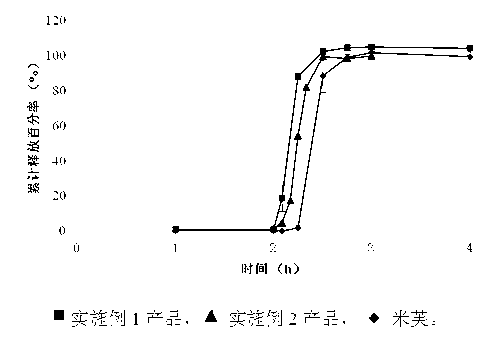
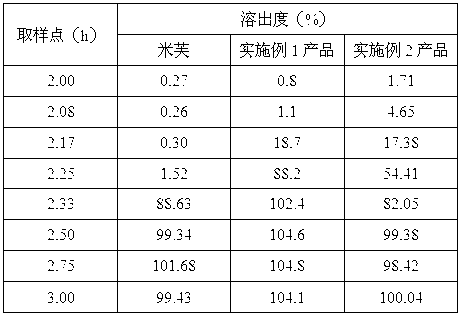
Image
Examples
Embodiment 1
[0046] prescription:
[0047] Sodium mycophenolate mofetil 192.4 mg
[0048] Acrylic resin No. Ⅱ 5 mg
[0049] Lactose 30 mg
[0050] Microcrystalline Cellulose 30 mg
[0051] Sodium carboxymethyl starch 40 mg
[0052] Magnesium stearate 5 mg
[0053] Silica 10 mg
[0054]Preparation method: Take the prescription amount of polyacrylic acid resin No. Ⅱ and dissolve it in an appropriate amount of absolute ethanol to prepare 0.05 g·mL -1 The solution, set aside; take the prescription amount of mycophenolate sodium mofetil, silicon dioxide, microcrystalline cellulose, lactose, and appropriate amount of sodium carboxymethyl starch in a mortar, grind and mix evenly, slowly add the above polyacrylic acid resin II No. ethanol solution, keep grinding until uniform, continue to drop an appropriate amount of absolute ethanol to prepare soft material; the obtained soft material is passed through a 18-mesh sieve to make granules, and dried at 40°C for 2 h; the obtained dry granules a...
Embodiment 2
[0056] prescription
[0057] Sodium mycophenolate mofetil 384.7 mg
[0058] Acrylic resin No. 20 mg
[0059] Lactose 65 mg
[0060] Sodium carboxymethyl starch 50 mg
[0061] Magnesium stearate 7 mg
[0062] Silica 13 mg
[0063] Preparation method: Take the prescription amount of polyacrylic acid resin No. Ⅱ and dissolve it in an appropriate amount of absolute ethanol to prepare 0.05 g·mL -1 The solution, set aside; take the prescription amount of sodium mycophenolate mofetil, silicon dioxide, lactose, and appropriate amount of sodium carboxymethyl starch in a mortar, grind and mix evenly, slowly add the above-mentioned polyacrylic acid resin No. Ⅱ ethanol solution dropwise, keep After grinding until uniform, continue to drop an appropriate amount of absolute ethanol to prepare soft material; the obtained soft material is passed through a 18-mesh sieve to make granules, and placed in 40°C for 2 h to dry; the obtained dry granules are mixed with the remaining sodium carbo...
Embodiment 3
[0065] prescription
[0066] Sodium mycophenolate mofetil 384.7 mg
[0067] Polyvinyl phthalate 50 mg
[0068] Lactose 65 mg
[0069] Croscarmellose sodium 23 mg
[0070] Magnesium stearate 7 mg
[0071] Silica 13 mg
[0072] Preparation method: Dissolve the prescription amount of polyvinyl alcohol phthalate in an appropriate amount of absolute ethanol to prepare 0.05 g·mL -1 Put the solution of mycophenolate mofetil, silicon dioxide, and lactose in the mortar, grind and mix evenly, slowly add the above-mentioned ethanol solution of polyvinyl alcohol phthalate, keep grinding until uniform, and then continue to drop Add an appropriate amount of absolute ethanol to prepare the soft material; pass the obtained soft material through a 18-mesh sieve to make granules, and dry them at 40°C for 2 hours; after mixing the obtained dry granules with croscarmellose sodium and magnesium stearate, It is compressed into tablets on a single tablet press, with a hardness of 7~10 kgf.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com