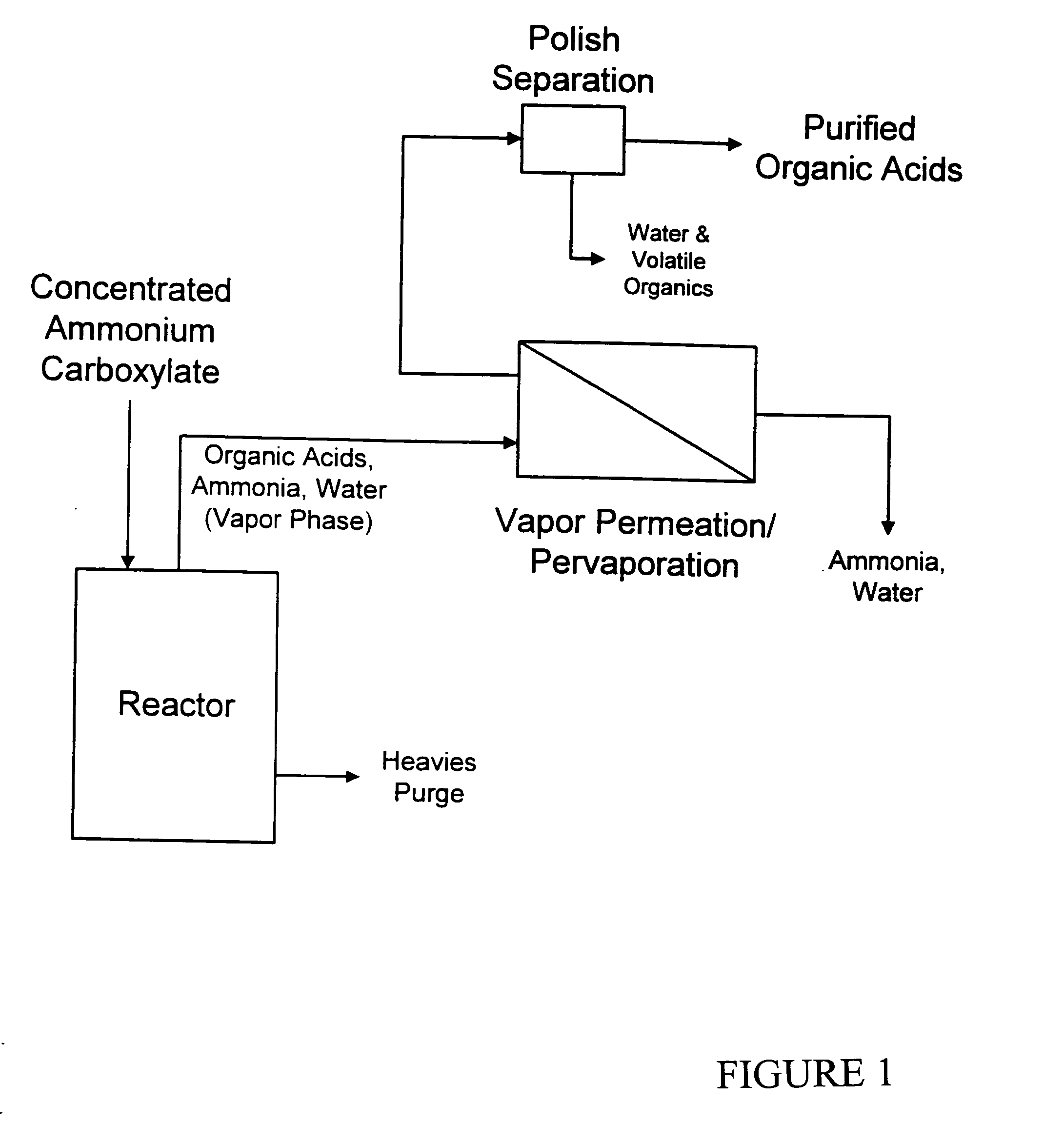
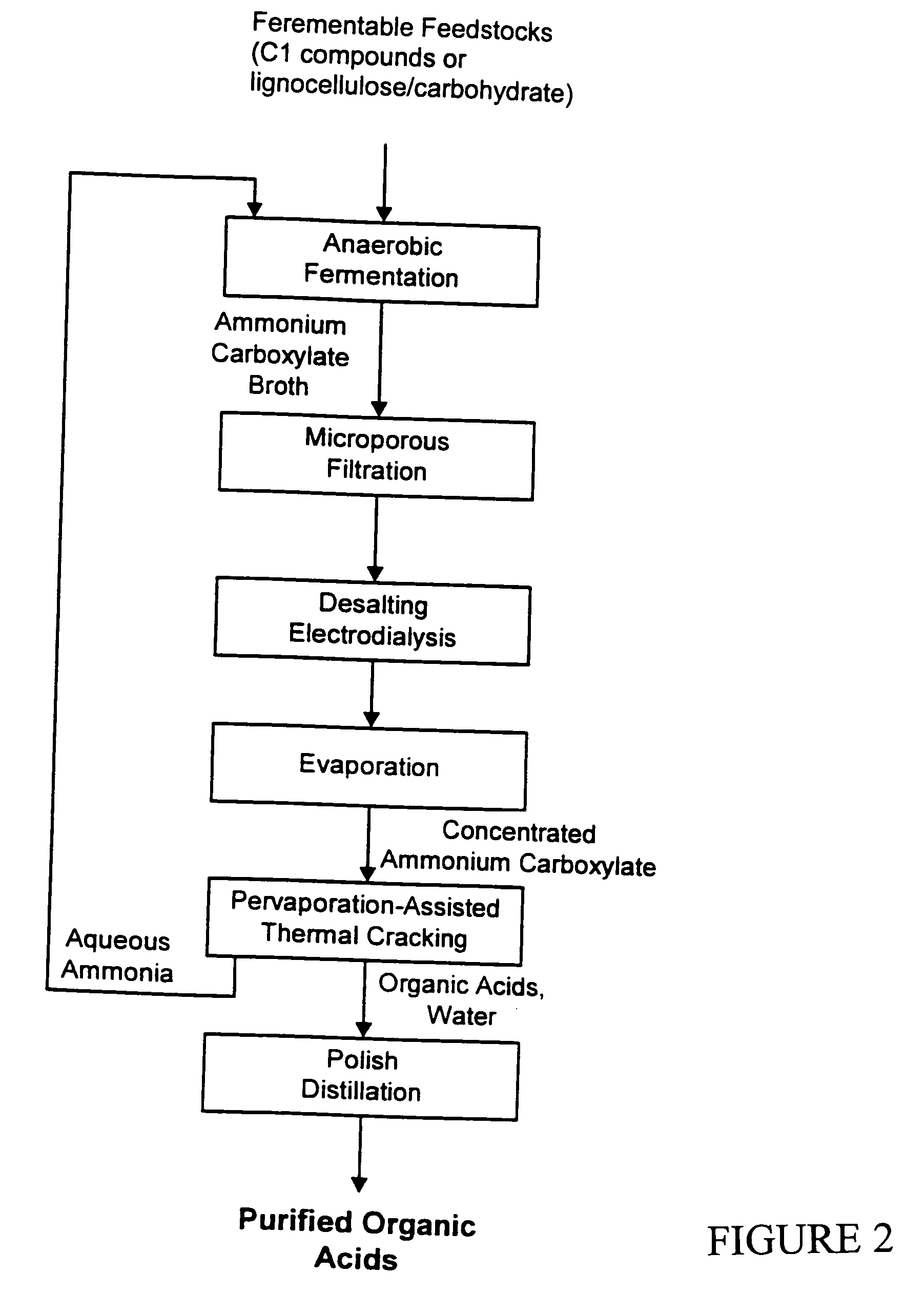
Processs for production and purification of fermentation derived organic acids
a technology of organic acids and purification processes, applied in the field of process for the production and purification of fermentation derived organic acids, can solve the problems of prohibitively expensive process for lower value acids, few separation processes have succeeded technically
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0015] A simple apparatus was set up to sublimate ammonium acetate solutions at controlled temperatures between 100° C. and 120° C. An HPLC based method was also developed to quantify acetic acid and acetamide concentrations.
[0016] Preliminary results from the initial experiments showed: [0017] 1a. The rate of sublimation increases with temperature and very good rates can be attained at a temperature of 120° C. [0018] 1b. Under these conditions of free sublimation of ammonium acetate solution, the rate of the byproduct acetamide formation is significantly lower than the rate of volatilization. In these experiments the ratio of rates were about 1:50 to 1:100. This means the kinetics are favorable for acetic acid formation and there is not a fundamental kinetic barrier to the development of a high yield separations process.
[0019] Further tests conducted at even higher temperatures of 125° C. and 140° C. in aclosed reactor showed that the rate of acetamide formation from an 80% w / w s...
example 2
[0022] The Sulzer membranes identified above were tested with liquid phase feed of ammonia, water and ethanol and found that one of the membrane types, Sulzer # 2211, had good water flux, and moderate ammonia flux and the ammonia fluxes increased considerably (˜2.5 fold) with temperature increase from 100° C. to 120° (Table 3).
TABLE 3Acid-Tolerant Membrane Flux ComparisonAll tests conducted with Sulzer Circular, Flat-SheetPervaporation Module in Liquid-Phase ModeReactorWaterConc.WaterRunSulzerAvg. ReactorReactor NH3NH3range,Flux,No.MembraneTemp., ° C.Conc. range, wt %Flux, kg / m2-hrwt %kg / m2-hr421201-D972.6-2.4%˜0.058.1-5.5%˜0.5431201-D1172.6-2.1%˜0.157.4-2.3%˜1.3501211-NV982.6-2.4%˜0.068.5-6.3%˜0.5511211-NV1202.6-2.1%˜0.207.0-2.6%˜1.25222111002.6-2.3%˜0.117.2-4.2%˜0.85322111202.5-1.8%˜0.287.0-1.8%˜1.4
[0023] A vapor permeation module was designed and assembled with #2211 membrane (0.022 m2 membrane area) and tested its performance with water, ethanol and ammonia vapor feed and esta...
example 3
[0024] For this experiment an 80% (w / w) ammonium acetate solution in water was prepared and heated in a closed reactor to 135° C. and allowed the pressure to build. At the same time the vapor permeation module with the #2211 membrane (0.022 m2) was preheated to ˜120° C. This was necessary to insure that no liquid acetic acid or water would condense on the membrane surface during the test run.
[0025] At the beginning of the run the vapor release valve at the top of the reactor was opened and after the vapor passed over the module it was condensed and collected in an enclosed condenser. The permeate from the module was condensed in a cold (0° C.) condenser and any uncondensed permeate vapors were collected in an acid trap (containing ˜25% sulfuric acid) and a cold trap (−50 C). The test run lasted for ˜15 minutes after which no more vapor was being produced by the reactor. Samples from the reactor, condensate, permeate, traps and the vapor were taken and carefully analyzed for free am...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Boiling point | aaaaa | aaaaa |
| Pressure | aaaaa | aaaaa |
| Stability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com