Expandable medical device for delivery of beneficial agent
a medical device and beneficial agent technology, applied in the field of tissue-supporting medical devices, can solve the problems of difficulty in accurately placing the stent or finding and retrieving stents that subsequently become dislodged and lost in the circulatory system, stents are often unstable, and display a tendency to buckle, etc., to eliminate buckling and twisting of structural features during stent deployment, and increase the available depth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
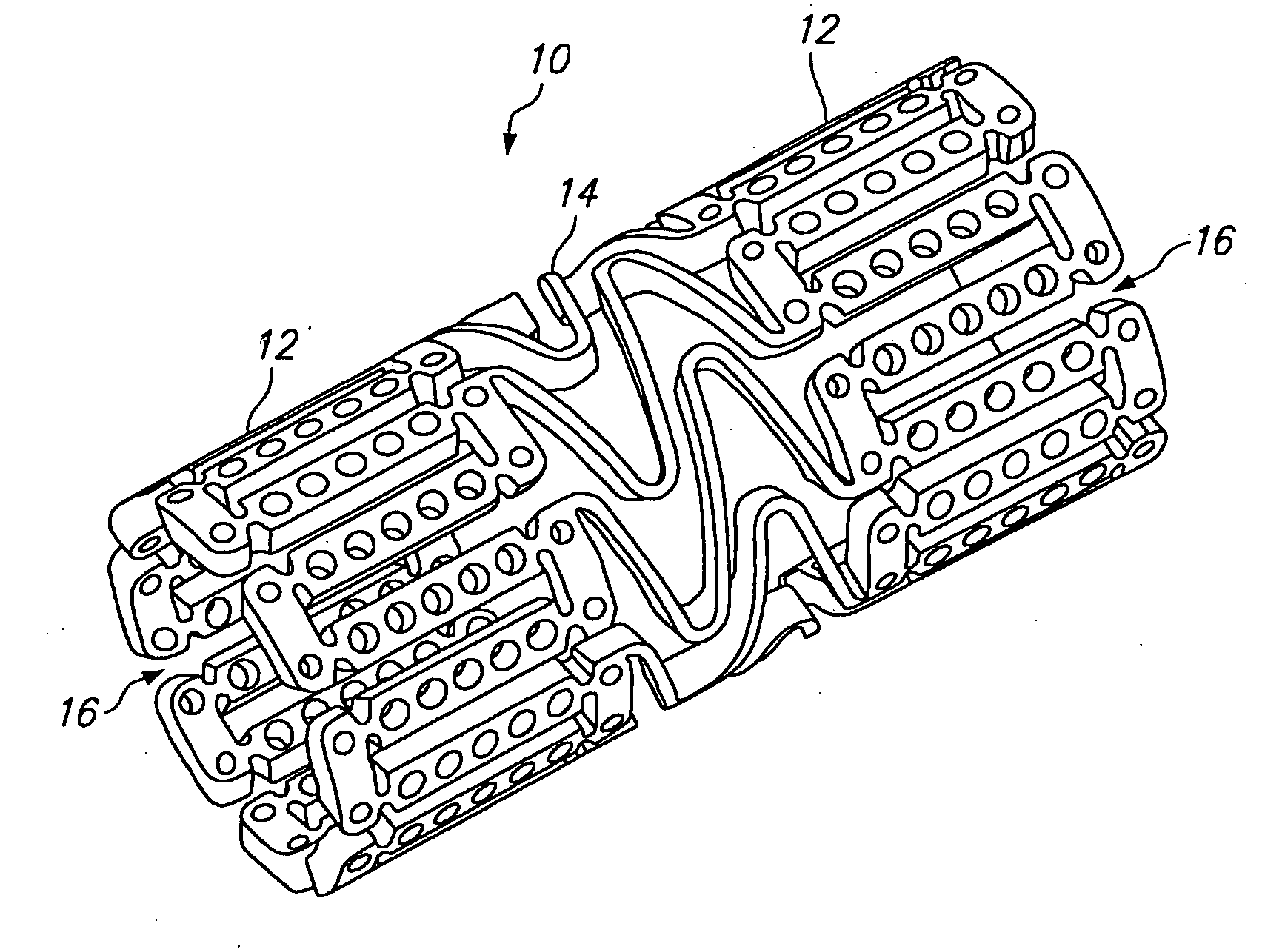
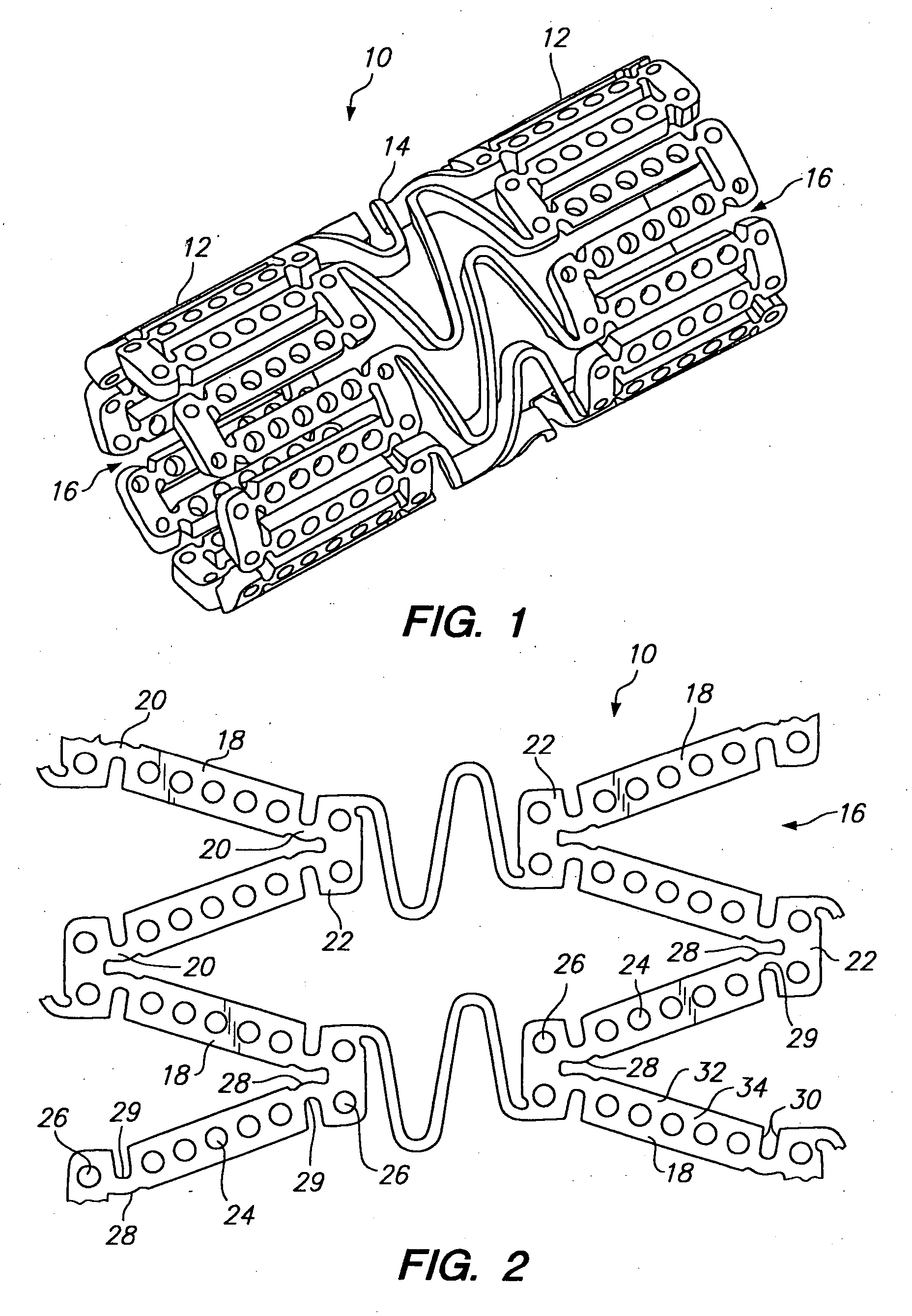
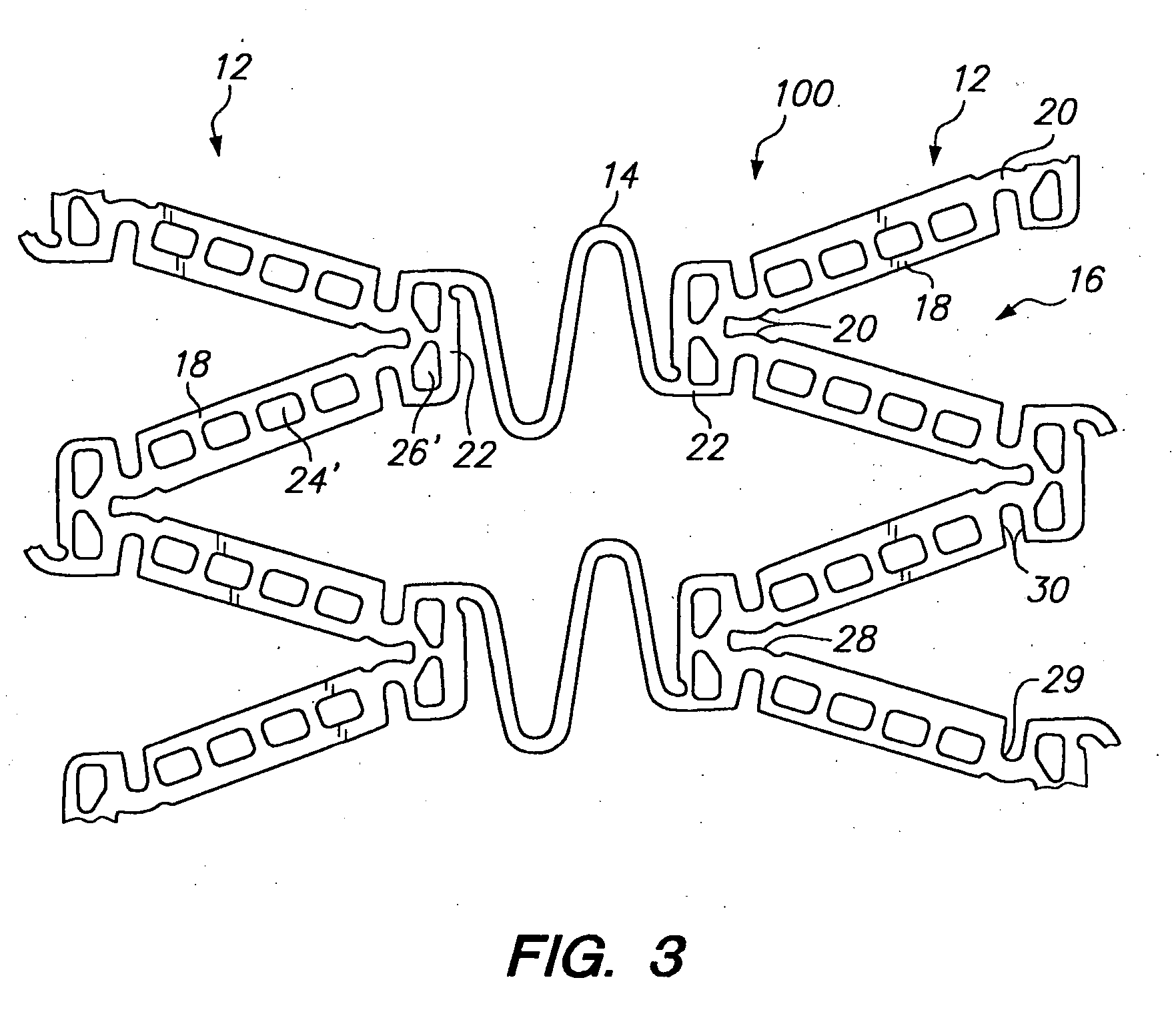
[0039] Referring to FIGS. 1 and 2, a tissue supporting device in accordance with a preferred embodiment of the present invention is shown generally by reference numeral 10. The tissue supporting device 10 includes a plurality of cylindrical tubes 12 connected by S-shaped bridging elements 14. The bridging elements 14 allow the tissue supporting device to bend axially when passing through the tortuous path of the vasculature to the deployment site and allow the device to bend when necessary to match the curvature of a lumen to be supported. The S-shaped bridging elements 14 provide improved axial flexibility over prior art devices due to the thickness of the elements in the radial direction which allows the width of the elements to be relatively small without sacrificing radial strength. For example, the width of the bridging elements 14 may be about 0.0015-0.0018 inches (0.0381-0.0457 mm). Each of the cylindrical tubes 12 has a plurality of axial slots 16 extending from an end surfa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pressure | aaaaa | aaaaa |
| pressures | aaaaa | aaaaa |
| pressures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com