Retinal cell grafts and instrument for implanting
a technology of retinal cells and instruments, applied in the field of retinal cell grafts and instruments for implanting, can solve the problems of destroying the cellular polarity and native organization of the donor retinal pigment epithelium, unable to achieve effective reconstruction methods, and unable to achieve the effect of regrowth of photoreceptor axons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Experimental Animals
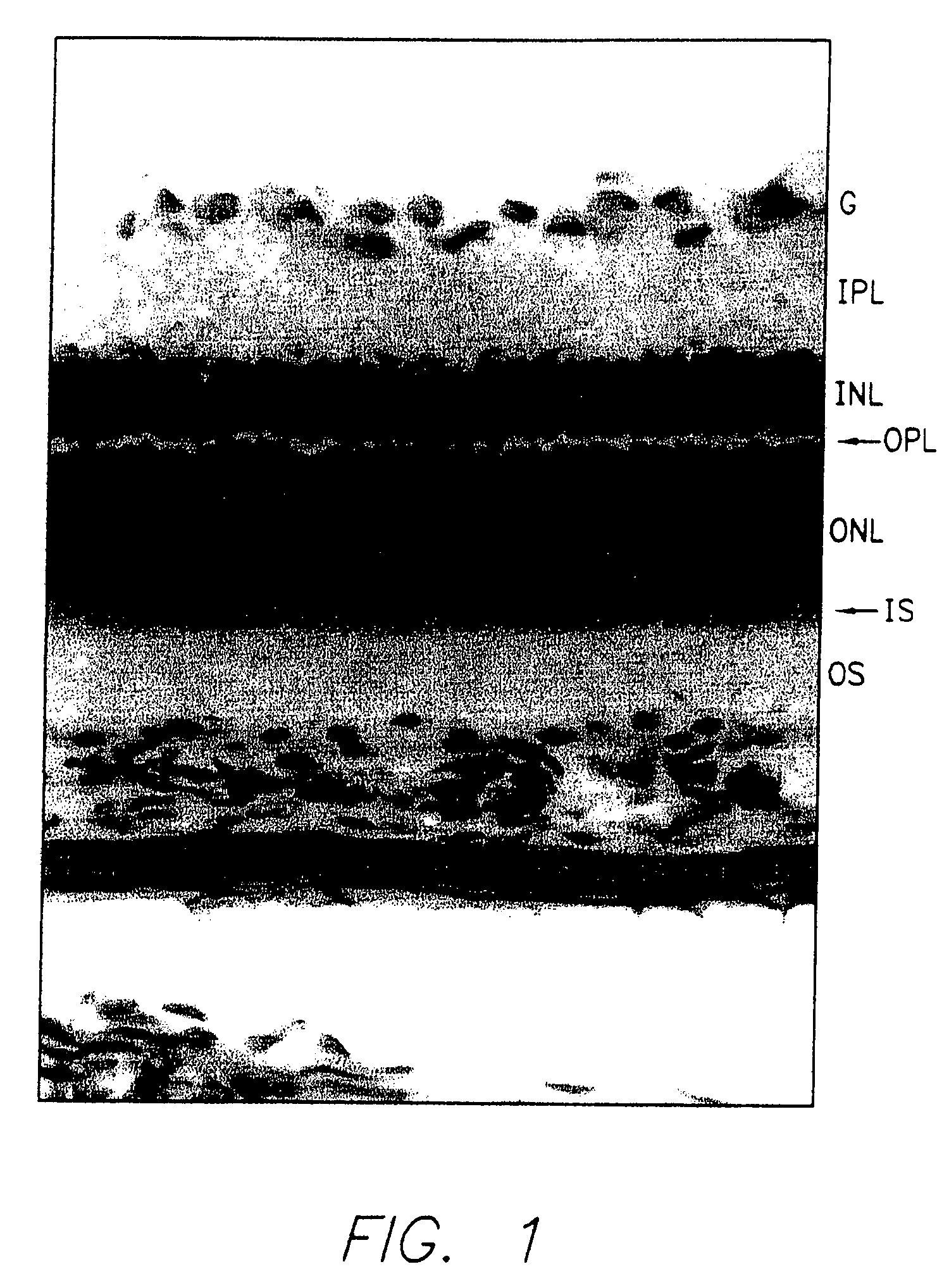
[0092] Adult albino rats (Sprague-Dawley) were exposed to constant illumination averaging 1900 lux for 2 to 4 weeks as described in O'Steen, Exp. Neurol. 27:194 (1970).
[0093] As shown in FIG. 1, this exposure destroys most photoreceptors, eliminating cells of the outer nuclear layer but leaving the remaining neural retina intact. Photoreceptors for transplantation were taken from 8-day-old normal rats of the same strain that had been maintained under colony room illumination (10-20 lux) on a 12 hr / 12 hr light / dark cycle. Experimental animals were anesthesized with ketamine and sodium pentobarbital. A preoperative dose of dexamethasone (10 mg / kg IP) was also administered.
Photoreceptor Preparation
[0094] The retina from the anesthetized 8-day-old rat was removed, flattened with radial cuts and placed with the receptor side down on a gelatin slab secured to the vibratome chuck. Molten gelatin (4-5% solution) was deposited adjacent the retina at the retina / gelat...
example 2
[0109] The procedures of Example 1 were repeated except as noted. Substituted for the Sprague-Dawley rats were the rd mouse and the RCS rat which are afflicted with inherited retinal degeneration. In the rd mouse it is thought that the deficit resides in the photoreceptor whereas in the RCS it is thought that the deficit resides in the pigment epithelium. In these animals, almost all photoreceptors are eliminated while the remaining retina is preserved; either the rd mouse or the RCS rat were blinded by constant illumination as set forth in Example 1. The rd mouse and the RCS respectively received transplants of immature (7-8 day old mouse or rat) and mature rat photoreceptors.
rd Mouse
[0110] The transplantation technique was adapted to the smaller size of the mouse eye. This modification allowed sheets of intact outernuclear layer to be transplanted to the subretinal space of the mouse eye. Neonatal (8 days old) photoreceptors were transplanted from rd control mice to the subreti...
example 3
[0115] The procedures of Example 1 were repeated except as noted.
[0116] Donor photoreceptors were originally harvested at the earliest ontogenetic time in which the photoreceptors could be isolated from other portions of the retina (7-8 days old) since it is generally believed that more embryonic and undifferentiated neural tissue survives transplantation far better than more mature and differentiated tissue. To determine the effect of developmental age on photoreceptor survival and ability to integrate with the host retina, photoreceptors were subsequently transplanted from 8, 9, 12, 15 and 30 day old rats into light damaged adults. These show progressive development and maturation of the photoreceptors including mature outer segments (at 15 and 30 days).
[0117] Using the same criteria as in Example 1, it was found that for all ages tested the transplants survived for as long as examined (2 months) and integrated with the host retina. FIG. 26, panel A, is a photograph of a transpl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| surface area | aaaaa | aaaaa |
| surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com