Accelerometer-based method for cardiac function and therapy assessment
a cardiac function and a catheter technology, applied in the field of catheters, can solve the problems of congestive cardiac failure affecting the synchronous beating of the ventricles, the risk of progressive heart failure or sudden death of patients with left ventricular failure, and the patient remains markedly symptomatic, so as to prevent further deterioration of cardiac function, reduce cardiac function, and improve cardiac function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
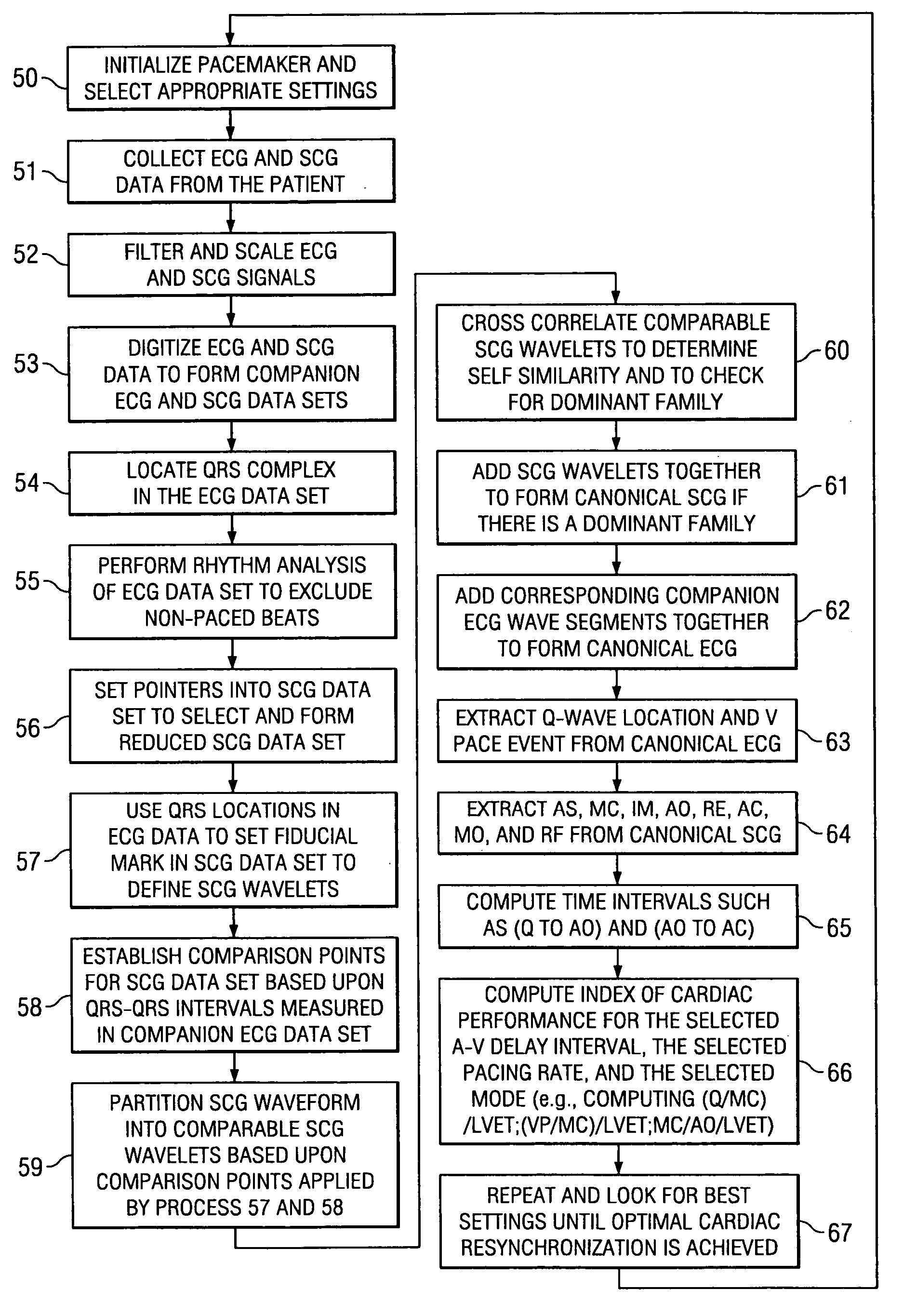
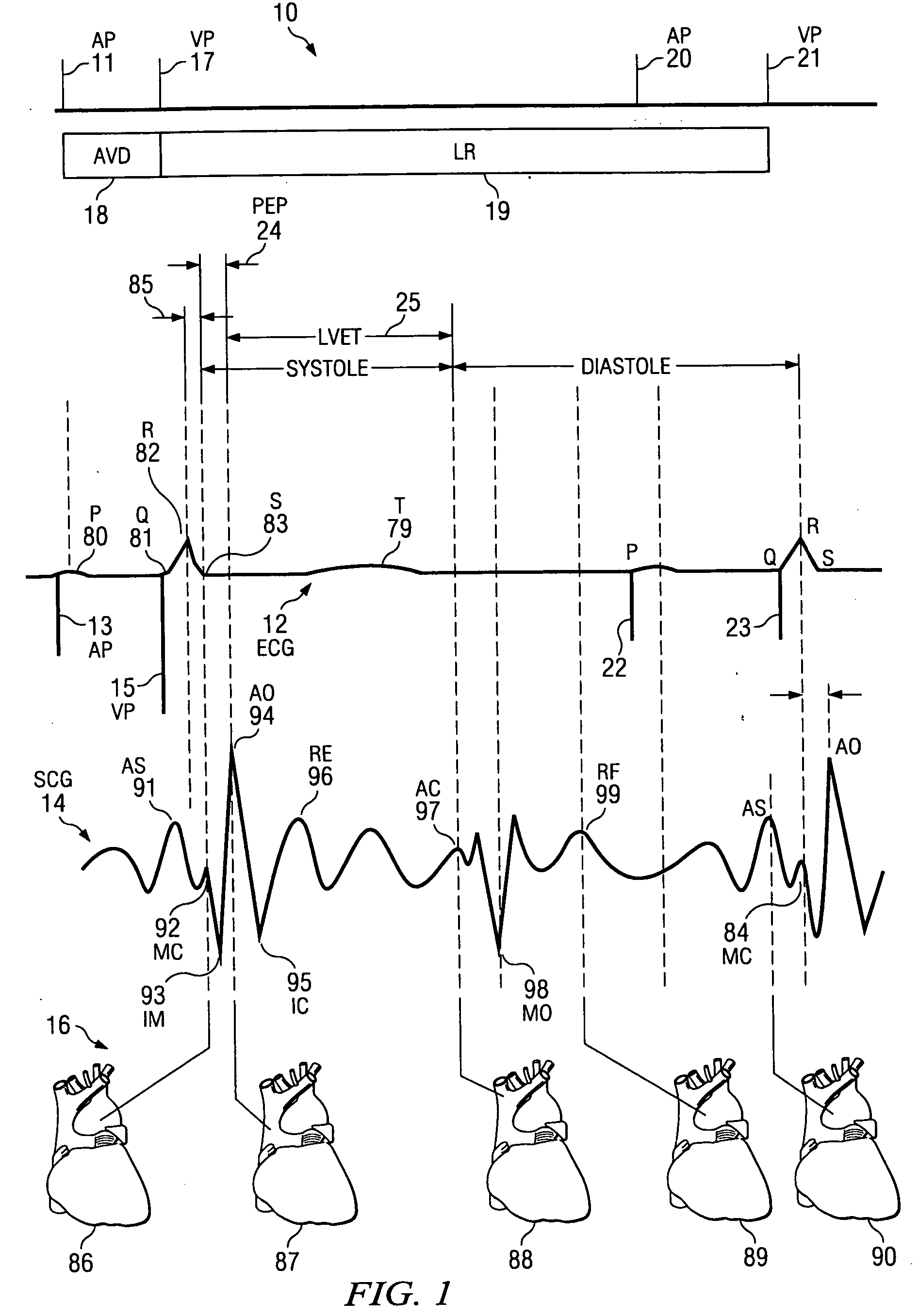
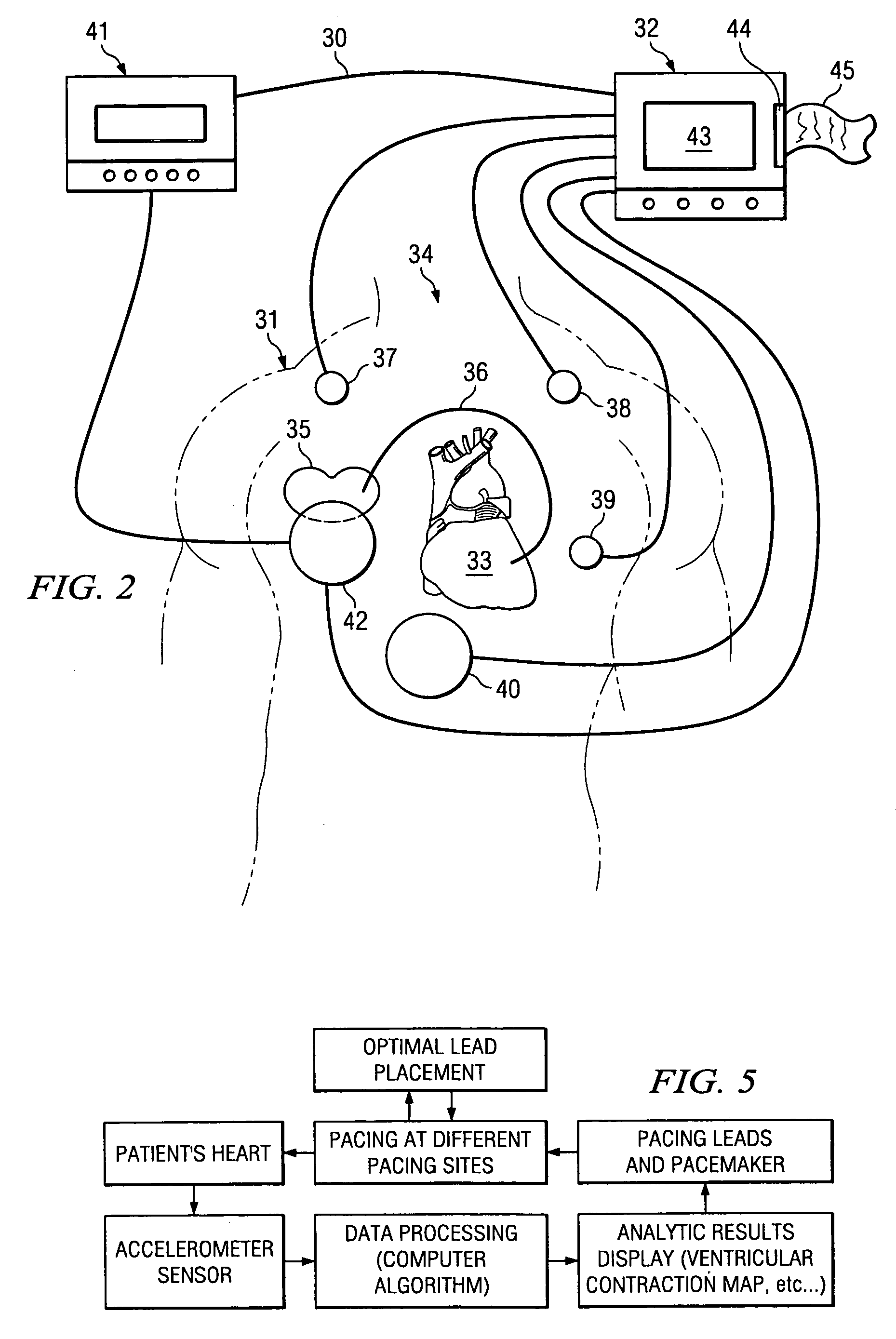
[0036] The invention relates in general to a method for determining a change in function of a patient's heart that includes the steps of collecting seismocardiographic (SCG) data from an external surface of a patient corresponding to a heart motion of the patient's heart; determining a hemodynamic parameter based on the SCG data; and comparing the parameter with a predetermined measure of cardiac performance.
[0037] Seismocardiography (SCG) is a method derived from the field of seismology to non-invasively measure compression waves generated by myocardial motion. These compression waves are measured by a low frequency accelerometer placed on the sternum, and recorded chest movement induced by ventricular motion is analyzed by software in a computer. The seismograph tracings identify cardiac time intervals that correlate with the opening and closing of cardiac valves, left ventricular (LV) systolic and diastolic time intervals, isovolumetric contraction and relaxation intervals, LV f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com