Immunologically privileged cells and uses thereof
a technology of immunodeficiency disease and privileged cells, applied in the field of immunodeficiency disease, can solve the problems of severe cushings-like hypercortisolemic syndrome, significant risk of serious long-term complications for patients with iddm, and limited clinical applicability of this approach
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
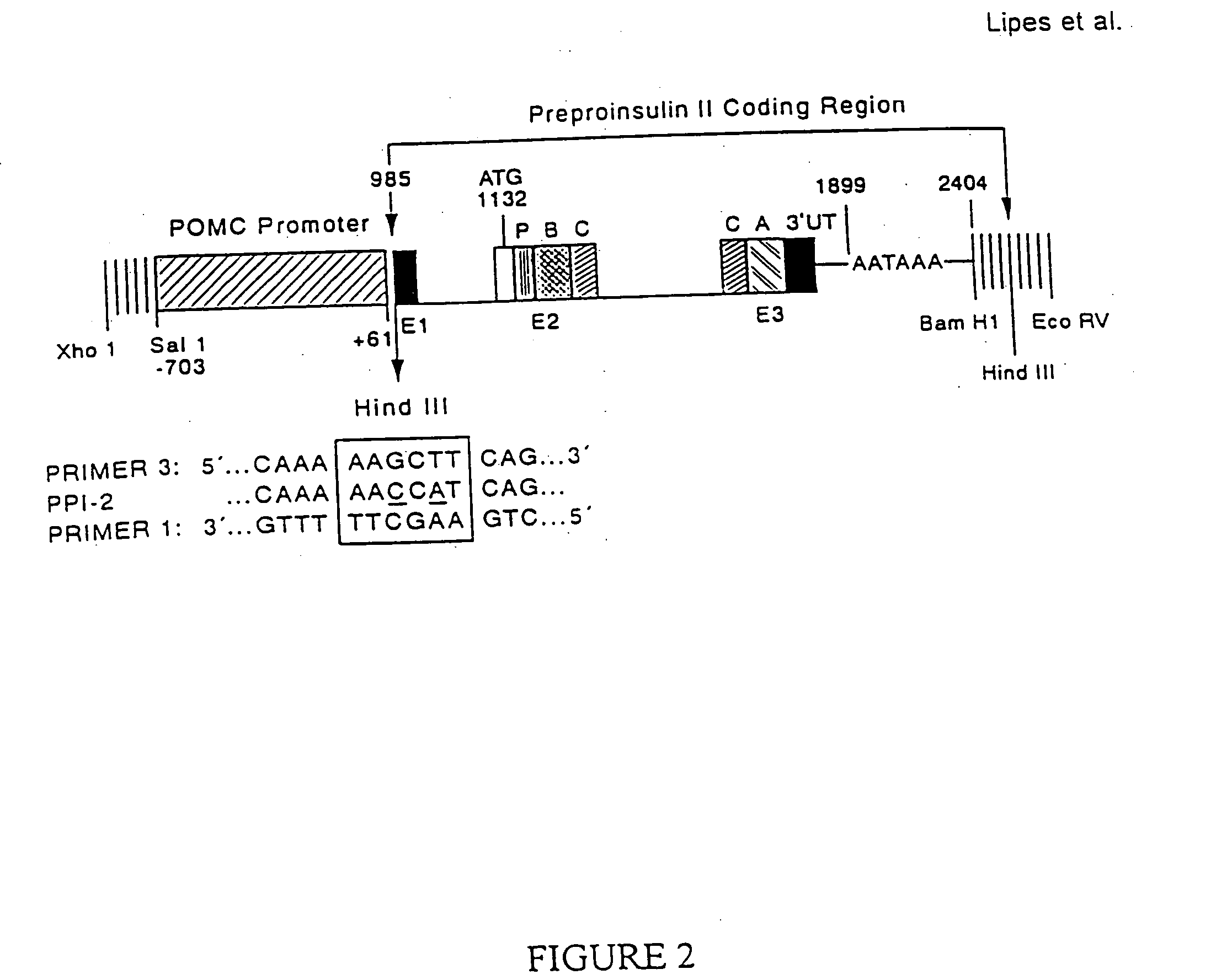
Construction of a POMC-Insulin Transgene
[0095] The POMC-Insulin transgene consisted of the POMC promoter region linked to the structural region of the mouse preproinsulin II (Ins) gene (FIG. 2). To excise the 5′ regulatory region of the Ins gene yet preserve the translation initiation start site at position 1132, a novel Hind III restriction site was created at position 985 by site-directed mutagenesis using the recombination polymerase chain reaction.(PCR) technique (Jones, D. H., Sakamoto, K., Vorce, R. L. & Howard, B. H. (1990) Nature (London) 344, 793-794). A 2.4 Kb genomic Bam HI Ins fragment (Wentworth, B. M., Schaefer, I. M., Villa-Komaroff, L. & Chirgwin, J. M. (1986) J. Mol. Evol. 23, 305-312) was cloned into pBluescript (pBS, Stratagene). The recombinant Ins-pBS vector was linearized in two separate restriction enzyme digestion reactions with Bal I (position 846) and PfiM I (position 1237). These templates were then amplified in two separate PCR reactions using primer 3: ...
example 2
Generation of the NOD Transgenic Mice
[0097] The POMC-Ins fusion gene cassette was excised from pBS by digestion with Xho I / Eco RV (FIG. 2). The cassette was purified for microinjection and was microinjected directly into the pronuclei of one-cell embryos of NOD mice (Lipes, M. A., Rosenzweig, A., Tan, K.-N., Tanigawa, G., Ladd, D., Seidman, J. G. & Eisenbarth G. S. (1993) Science 259 1165-1169). Founders were identified by PCR and Southern blot analysis of tail DNA. One transgenic NOD line (POMC-Ins1) was studied in detail and is described herein.
example 3
Expression of Insulin in the Pituitaries of Transgenic NOD Mice
[0098] Northern blot analysis of RNA from pituitary cells revealed an abundant 550 bp insulin transcript, identical in size to the endogenous pancreatic insulin transcript. In contrast, RNA from transgenic hypothalamus, brain, thymus, spleen, lymph nodes, testes, kidney, liver and salivary gland failed to show insulin signal. Immunocytochemistry of the pituitaries from the transgenic animals showed that a small percentage of cells in the anterior lobe and the great majority of cells in the intermediate lobe stained positive for insulin, similar in distribution to ACTH immunostaining. The posterior pituitary was devoid of specific insulin immunostaining and showed background signals similar to nontransgenic control pituitaries. Colocalization of insulin and ACTH (or POMC) immunoreactivity to the same pituitary cells was demonstrated by double immunolabelling the same frozen section. The ACTH antibody used in these studie...
PUM
| Property | Measurement | Unit |
|---|---|---|
| conductance | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com