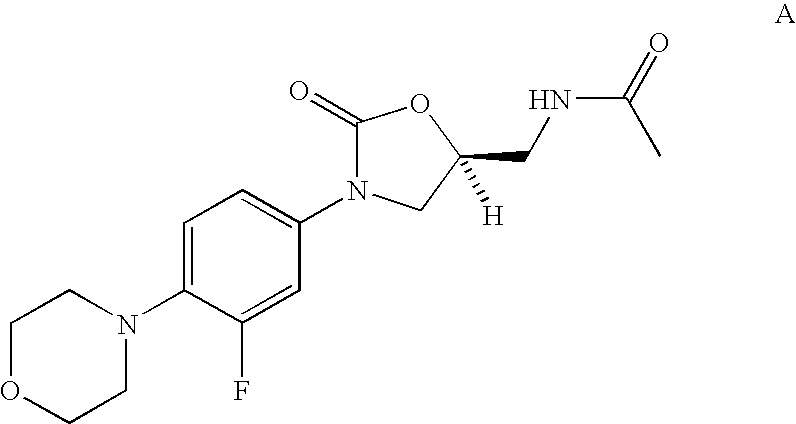
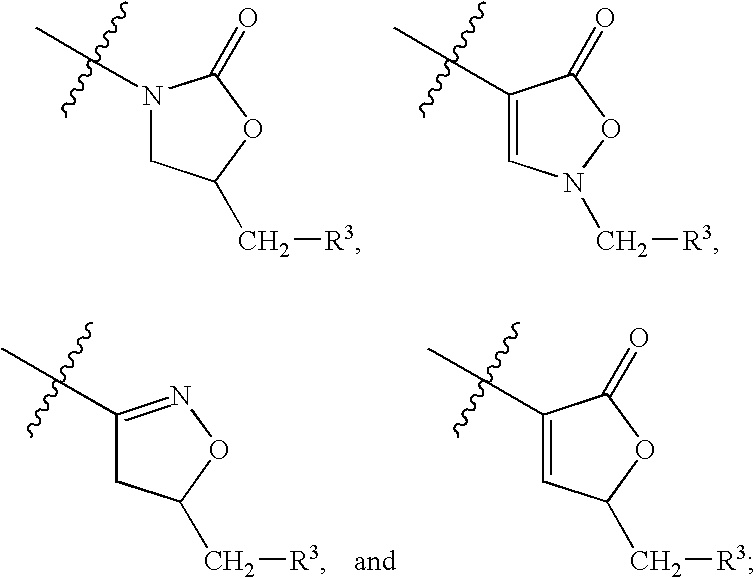
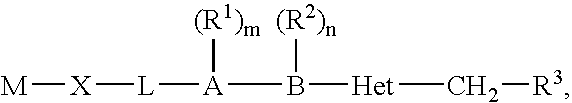Halogenated biaryl heterocyclic compounds and methods of making and using the same
a technology of biaryl heterocyclic compounds and biaryl heterocyclic compounds, which is applied in the field of antiinfective, antiproliferative, antiinflammatory, and prokinetic agents, can solve the problems of drug compositions, serious and even fatal results for patients infected with such resistant bacteria, and antibiotic agents developed for clinical use ultimately encounter problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Method A
[0134] Scheme 1 depicts the synthesis of aryl iodide 101, an intermediate useful in producing compounds of the present invention.
Synthesis of Amine 91
[0135] A solution of 3-fluoroanaline 90 (18.7 g, 168.3 mmol) in tetrahydrofuran (THF, 150 mL) was treated with potassium carbonate (K2CO3, 46.45 g, 336.6 mmol, 2.0 equiv) and water (H2O) (150 mL) before a solution of benzyl chloroformate (CBZCl, 31.58 g, 185.1 mmol, 26.1 mL, 1.1 equiv) in THF (50 mL) was dropwise added into the reaction mixture at room temperature under nitrogen (N2). The resulting reaction mixture was stirred at room temperature for 2 hours (h). When thin layer chromatography (TLC) showed the reaction was complete, the reaction mixture was treated with H2O (100 mL) and ethyl acetate (EtOAc, 100 mL). The two layers were separated, and the aqueous layer was extracted with EtOAc (2×100 mL). The combined organic extracts were washed with H2O (2×100 mL) and saturated aqueous sodiu...
example 2
Synthesis of Compounds 1 and 2
[0148] Scheme 3 illustrates the synthesis of amine 105, an intermediate used in the synthesis of compounds 1 and 2. Aryl iodide 101 is coupled to a substituted aryl boronic acid (the Suzuki reaction) to produce N-[3-(2-fluoro-4′-hydroxymethyl-biphenyl-4-yl)-2-oxo-oxazolidin-5-ylmethyl]-acetamide (biaryl alcohol 102). Other coupling reactions (for example, the Stille reaction), using alternate coupling intermediates easily obtained or synthesized by those skilled in the art, could also be employed to synthesize target biaryl intermediates similar to biaryl alcohol 102. Biaryl alcohol 102 is then converted to amine 105 by chemistry well known to those skilled in the art.
[0149] Scheme 4 illustrates the synthesis of compounds 1 and 2. Amine 105 is alkylated with the requisite bromides to afford compounds 1 and 2.
Synthesis of Amine 105
[0150] A suspension of aryl iodide 101 (14.0 g, 37 mmol) in toluene (120 mL) was treated with 4-(hydroxymethyl)phenylb...
example 3
Synthesis of Amine 3
[0156] Scheme 5 illustrates the synthesis of amine 3. 3-fluoropropylamine hydrochloride (amine hydrochloride 106) was synthesized from 1-bromo-3-fluoropropane 139 and then alkylated with benzyl chloride 107 to afford amine 3.
Synthesis of Benzyl Chloride 107
[0157] To a solution of 3-(3-fluoro-4-iodo-phenyl)-5-hydroxymethyl-oxazolidin-2-one (1.0 g, 2.97 mmol), isoxazol-3-ol (0.30 g, 3.56 mmol) and triphenylphosphine (1.02 g, 3.86 mmol) in THF (15 mL) was added diisopropyl azodicarboxylate (DIAD, 0.74 mL, 3.56 mmol) dropwise at 0° C. The reaction was warmed to room temperature and stirring was continued for 2 h. The solvent was evaporated and the crude was purified on silica gel, eluting with hexanes / EtOAc 2:1 to 3:2, to give 3-(3-fluoro-4-iodo-phenyl)-5-(isoxazol-3-yloxymethyl)-oxazolidin-2-one (1.01 g; 84% yield).
[0158] A degassed mixture containing 3-(3-fluoro-4-iodo-phenyl)-5-(isoxazol-3-yloxymethyl)-oxazolidin-2-one (1.0 g, 2.48 mmol), 4-(hydroxymethyl)ph...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com