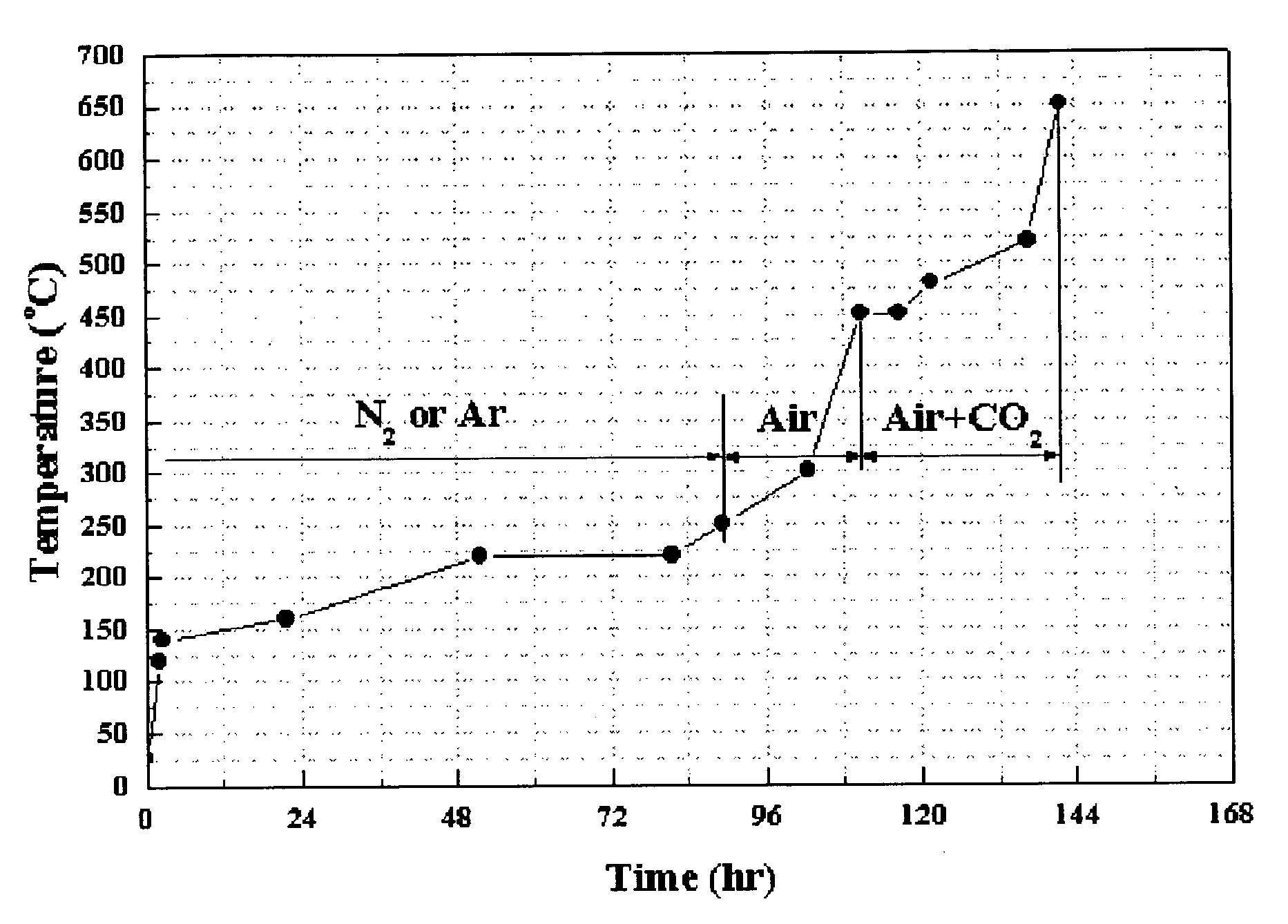
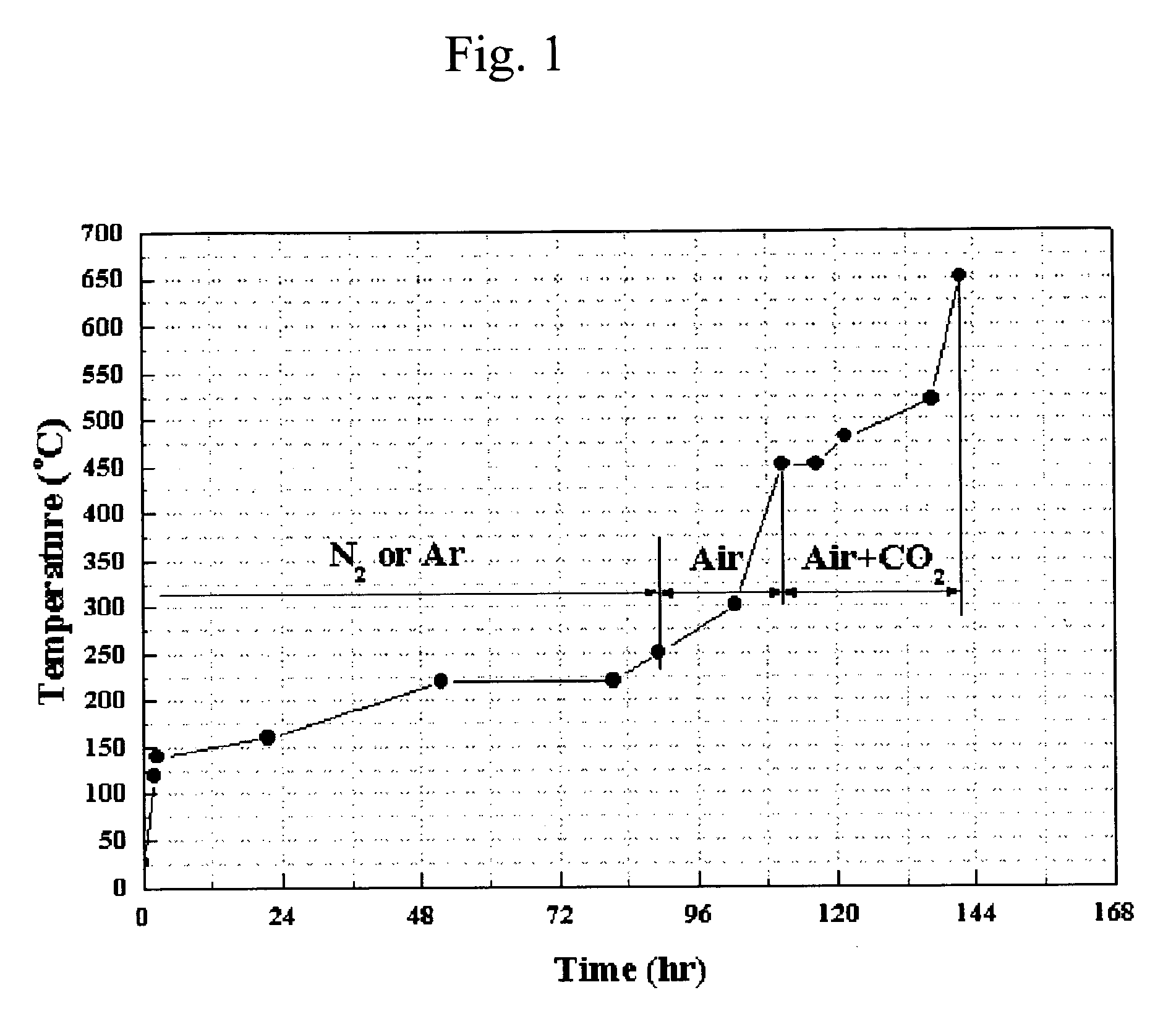
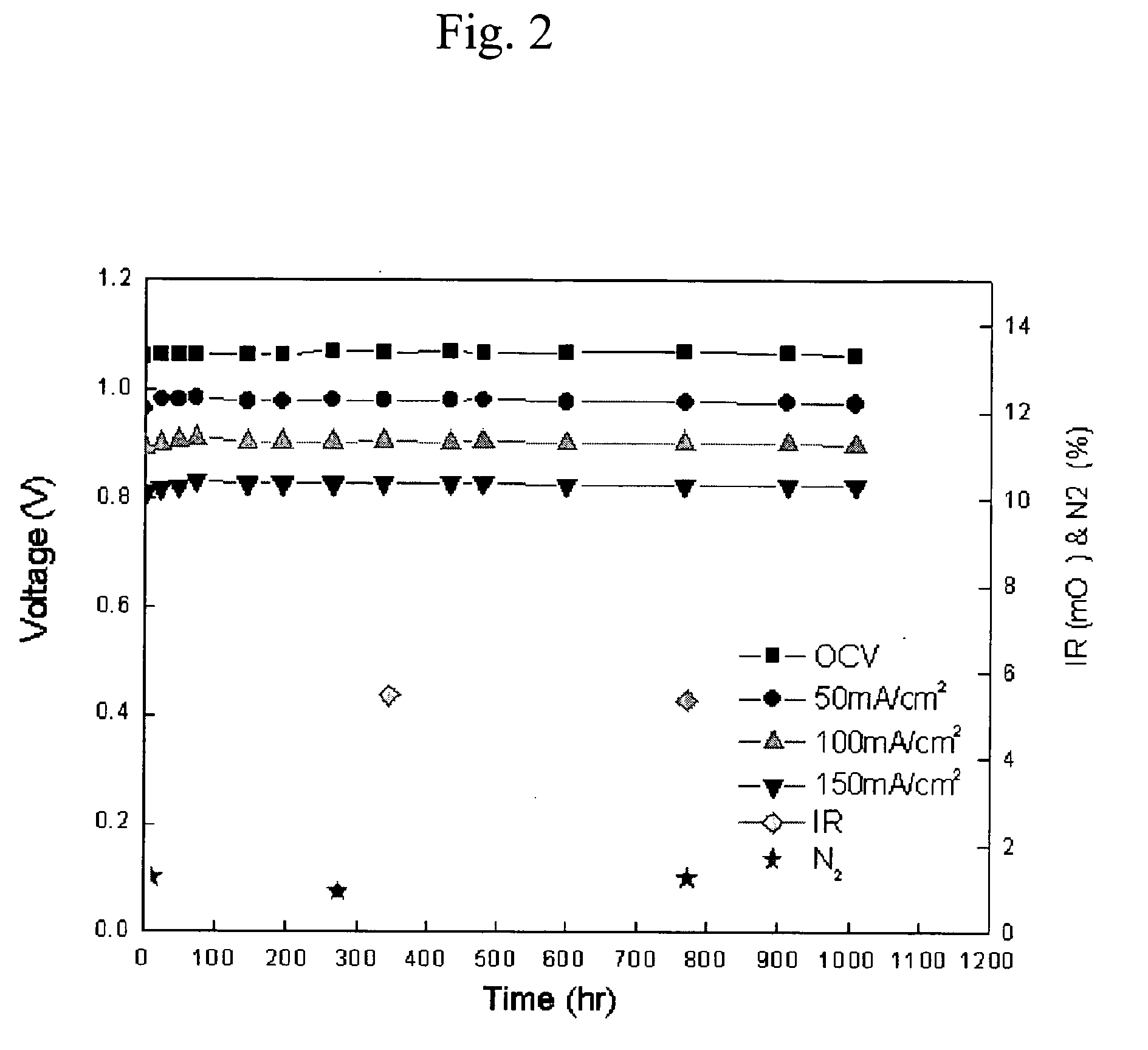
Ni-Al alloy anode for molten carbonate fuel cell made by in-situ sintering the Ni-Al alloy and method for making the same
a fuel cell and in-situ sintering technology, which is applied in the direction of cell components, final product manufacturing, sustainable manufacturing/processing, etc., can solve the problems of sintering creep, degradation of fuel cell performance, porous nickel electrodes, etc., and achieve simple and economic methods.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
[0030] A tape of nickel-aluminum alloy powders (i.e., a green sheet) was prepared as follows:
[0031] At first, a binder, a solvent, a plasticizer and a defoamer were primary-mixed and ball-milled for 24 hours, and then nickel-aluminum alloy powders (5 wt % or less aluminum) and a disperant were secondary-mixed and ball-milled for 2˜48 hours, thereby making a slurry.
[0032] Methyl cellulose 1500 (Hayashi Pure Chemical) was used as the binder. Water was used as the solvent. Glycerol (Junsei Chemical) was used as the plasticizer. SN 154 (San Nopco Korea) and Cerasperse 5468 (San Nopco Korea) were used as the defoamer and the disperant, respectively.
[0033] Based on 100 g of the nickel-aluminum alloy powders, 1˜2 g of the binder, 40˜50 g of the solvent, 1˜2 g of the plasticizer, 0.1˜1 g of the defoamer and 0.1˜1 g of the disperant were respectively used.
[0034] It could be checked through a slurry deposition experiment that the materials added when performing the second ball-mill were u...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| creep strain | aaaaa | aaaaa |
| creep strain | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com