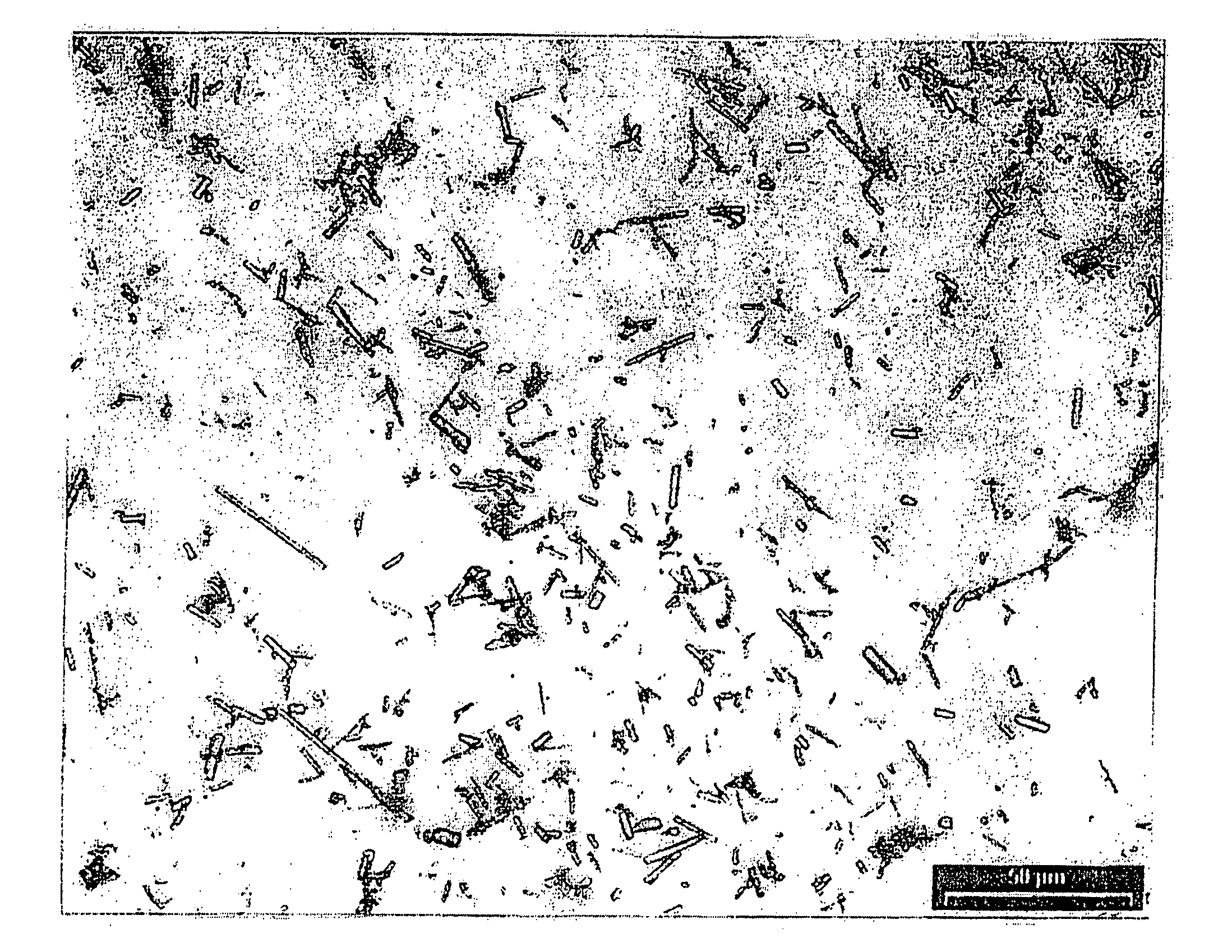
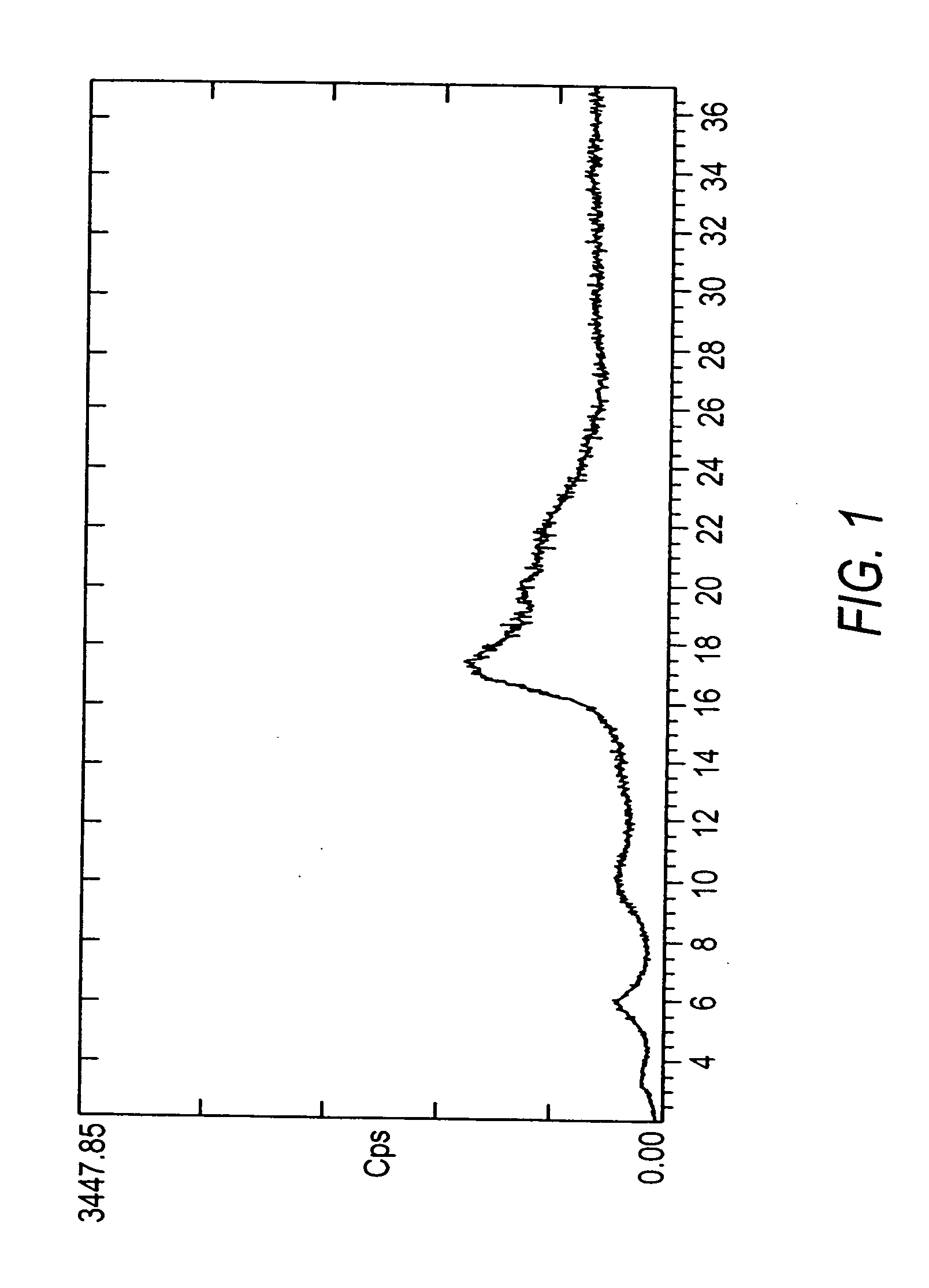
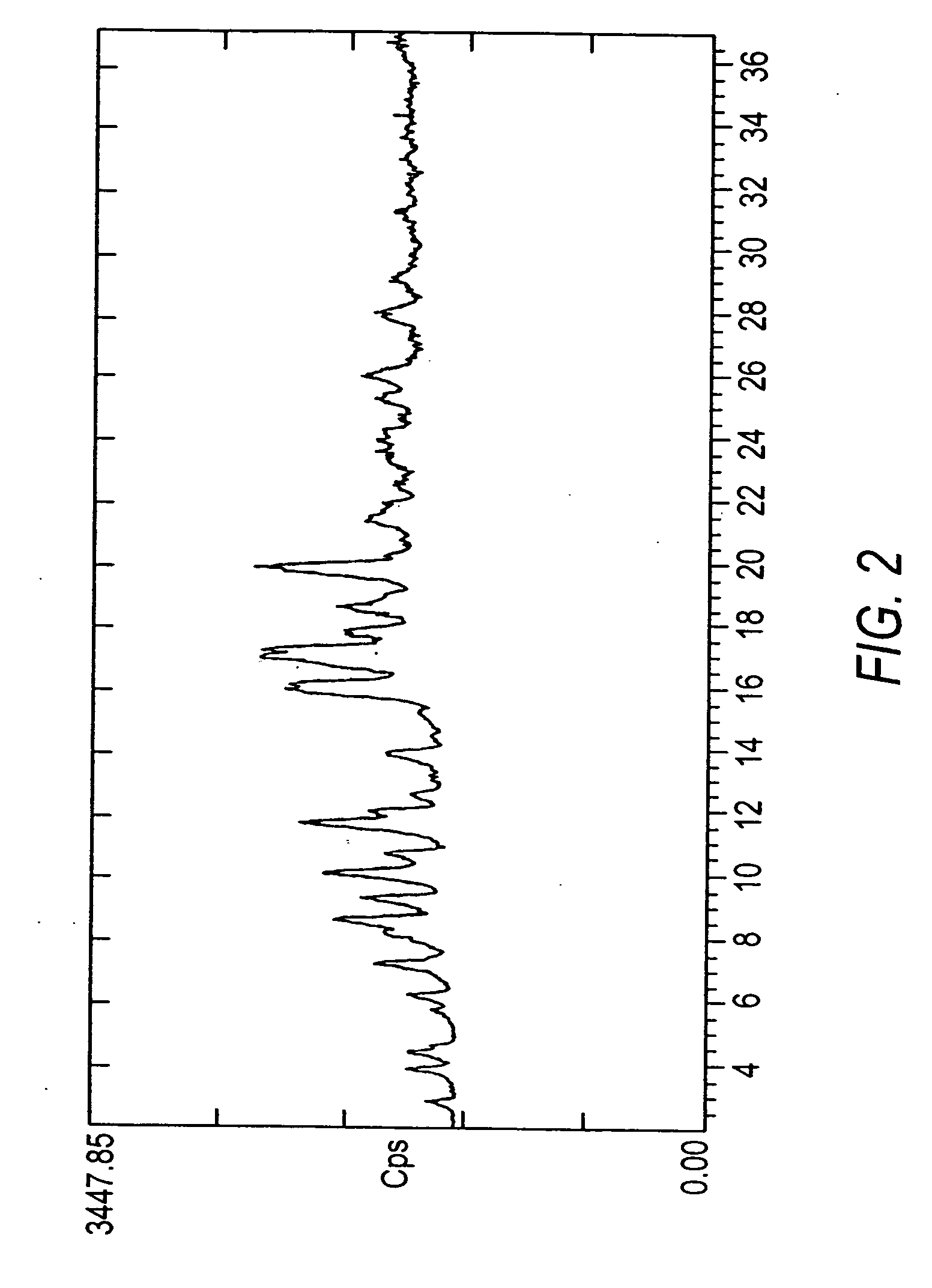
Crystals of the sodium salt of pravastatin
a sodium salt and sodium salt technology, applied in the field of crystallized form of the sodium salt of pravastatin, can solve the problems of not being economical in large-scale production operations for lyophilisation, and achieve the effect of improving purity and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0052] The sodium salt of pravastatin (1 g) was dissolved in methanol (10 ml) and while stirring ethyl acetate was added. The resulting clear yellow solution was cooled to 8° C. and allowed to stand overnight. Formed radiating clusters of thin, long needle-like crystals were filtered, washed with ethyl acetate (20 ml) and dried. Yield: 0.87 g of pale yellow crystals, melting point 172-174° C.
example 2
[0053] The sodium salt of pravastatin (2 g) was dissolved in methanol (20 ml) and while stirring ethyl acetate (80 ml) was added. The clear, slightly yellow solution was cooled to 8° C. and allowed to stand for 4 hours. Formed radiating clusters of thin, long needle-like crystals were filtered, washed with ethyl acetate (20 ml) and dried. Yield: 1.53 g of colorless crystals, melting point 172-174° C.
example 3
[0054] 20 The sodium salt of pravastatin (2 g) was dissolved in methanol (20 ml) and while stirring ethyl acetate (150 ml) was added. The resulting clear, slightly yellow solution was cooled to 8° C. and allowed to stand for 4 hours. Formed radiating clusters of thin, long needle-like crystals were filtered, washed with ethyl acetate (20 ml) and dried. Yield: 1.66 g of colorless crystals, melting point 172-174° C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com