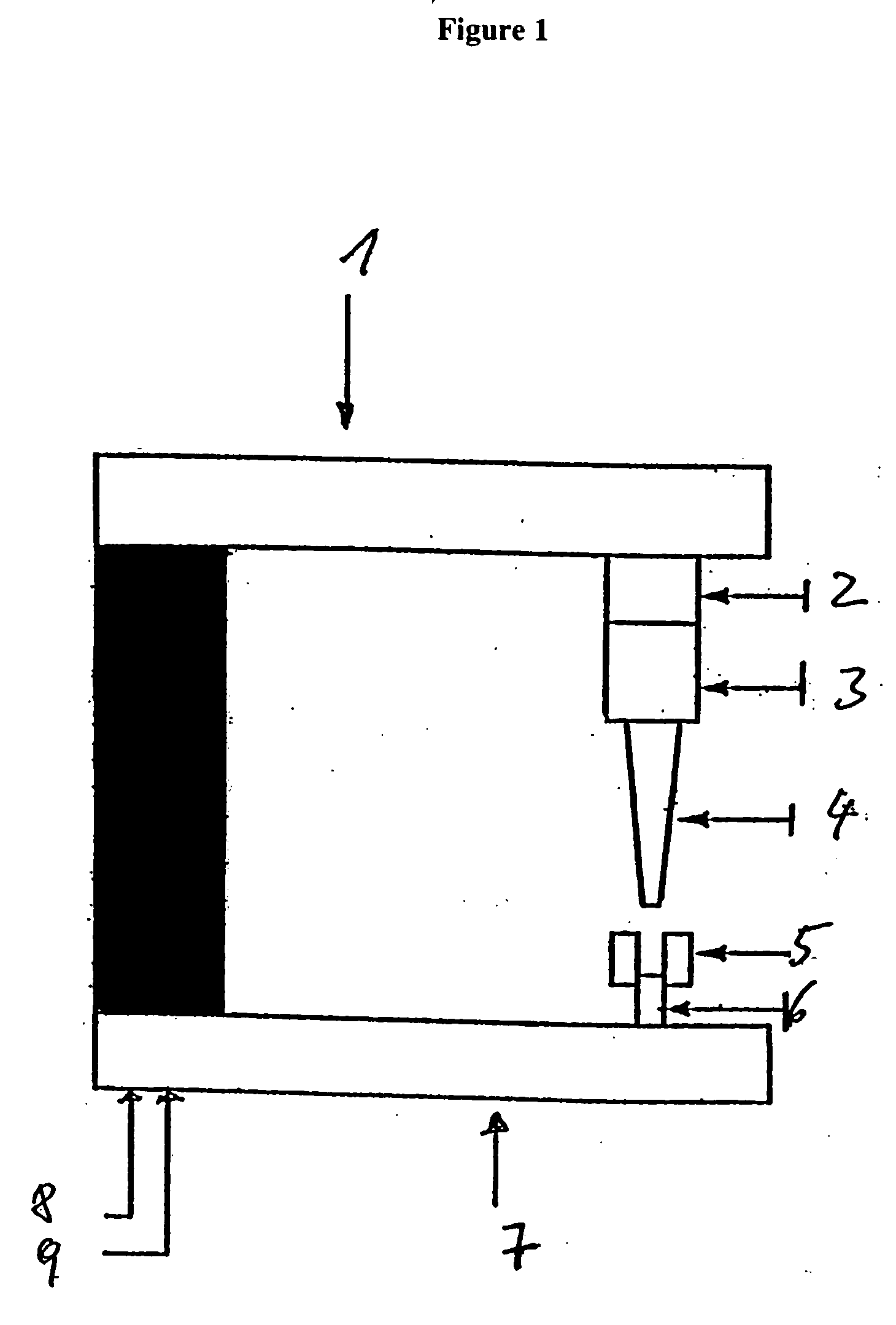
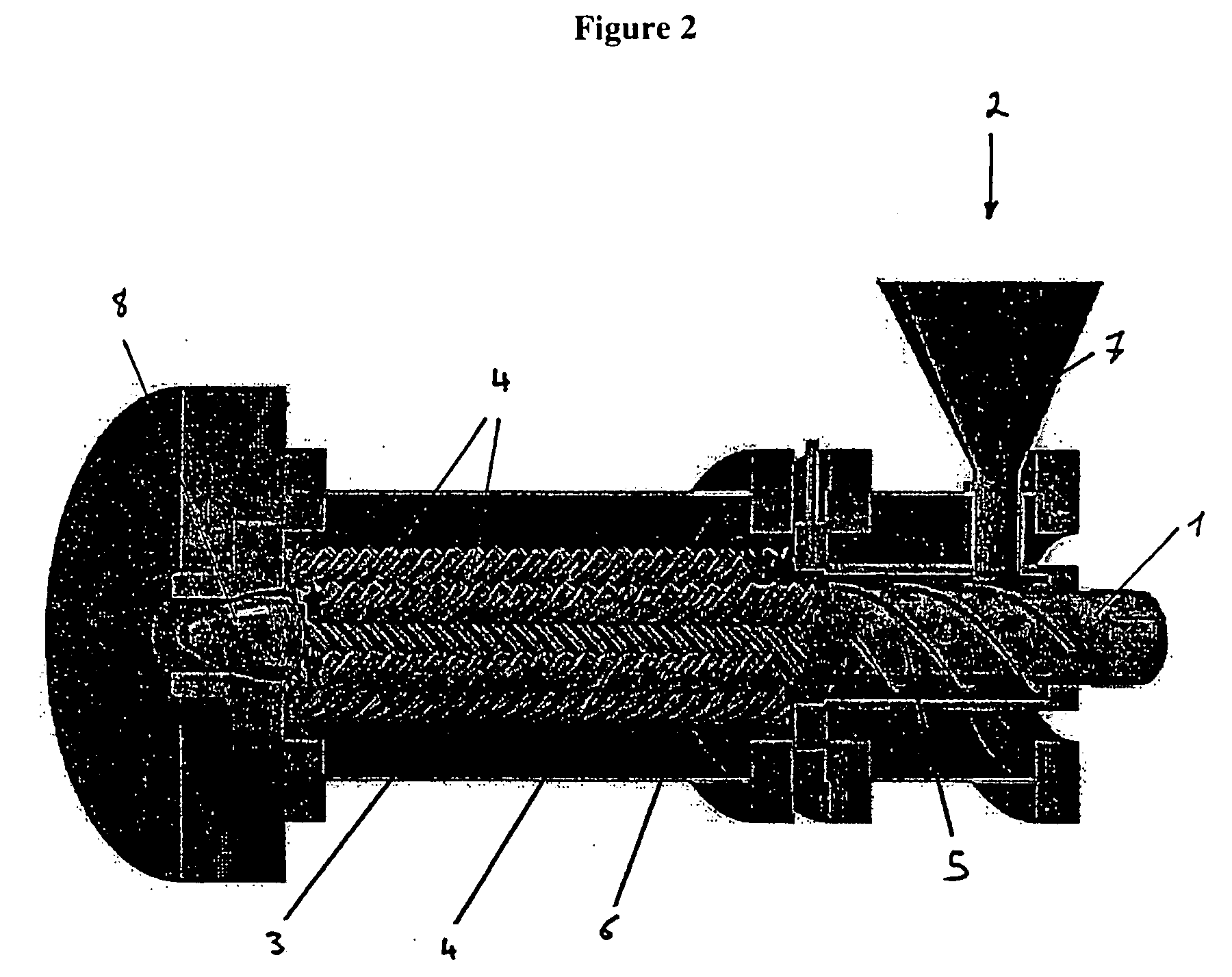
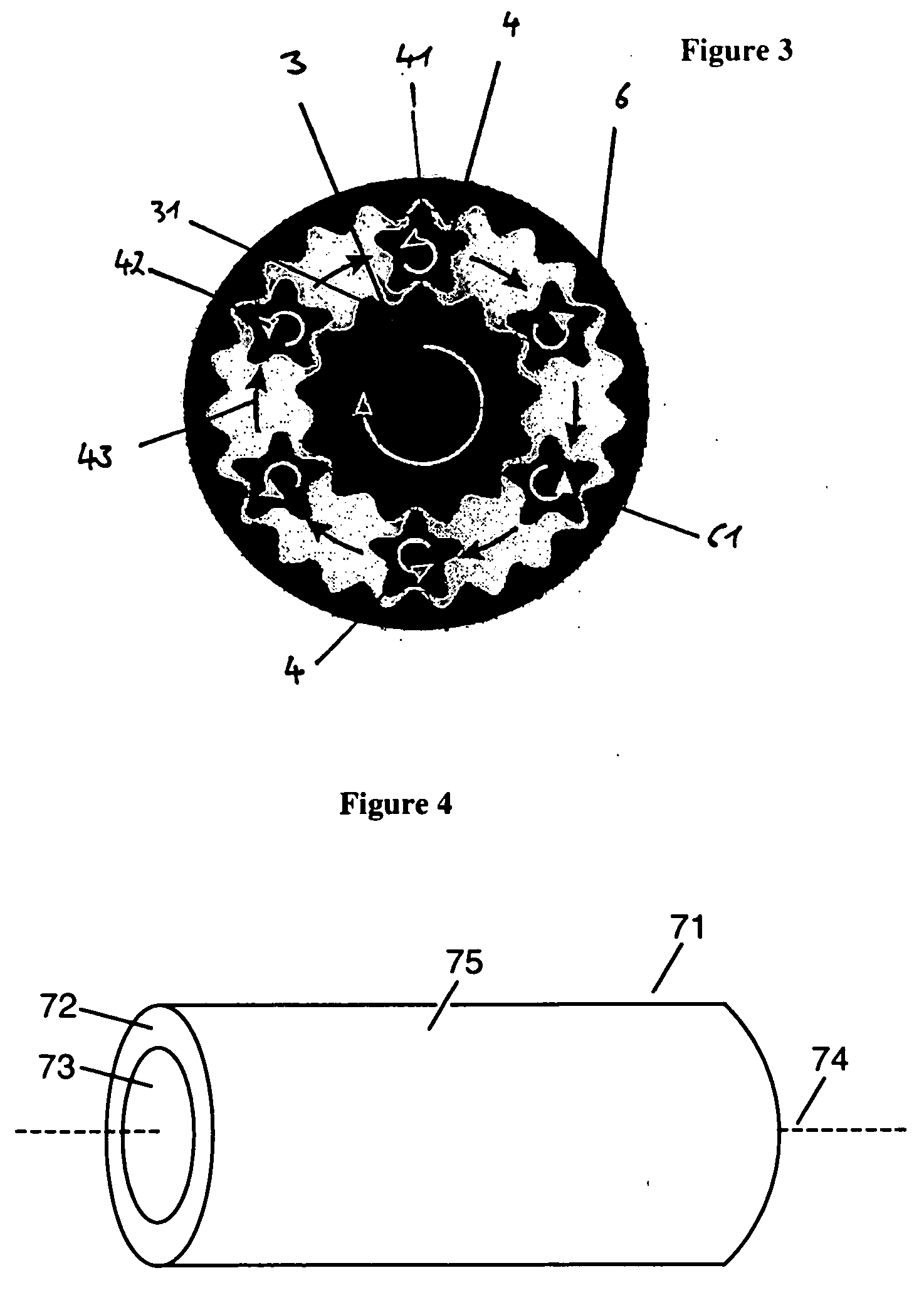
Crush resistant delayed-release dosage forms
a technology of delayed release and dosage form, which is applied in the field of dosage form, can solve the problems of difficult comminution of dosage form with such apparatus, affecting the safety of patients, and general unsuitability of pharmaceutical substances for “immediate release” formulations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
process embodiment 1
[0169] In this embodiment, the dosage form according to the invention is preferably produced without using an extruder by preferably mixing components (A), (C), optionally (B) and the optionally present component (D) and, optionally after granulation, shaping the resultant mixture by application of force to yield the dosage form with preceding and / or simultaneous exposure to heat.
[0170] This heating and application of force for the production of the dosage form proceeds without using an extruder.
[0171] Components (A), (C), optionally (B) and optionally (D) are mixed in a mixer known to the person skilled in the art. The mixer may, for example, be a roll mixer, shaking mixer, shear mixer or compulsory mixer.
[0172] The resultant mixture is preferably directly shaped into the dosage form according to the invention by application of force with preceding and / or simultaneous exposure to heat. The mixture may, for example, be formed into tablets by direct tabletting. In direct tablettin...
process embodiment 2
[0178] In this process variant, the dosage form according to the invention is produced by thermoforming with the assistance of an extruder, without there being any observable consequent discoloration of the extrudate.
[0179] In order to investigate the extent of discoloration due to this thermoforming, the color of the mixture of starting components of which the dosage form consists is first determined without addition of a color-imparting component, such as for example a coloring pigment or an intrinsically coloured component (for example α-tocopherol). This composition is then thermoformed according to the invention, wherein all process steps, including cooling of the extrudate, are performed under an inert gas atmosphere. By way of comparison, the same composition is produced by the same process, but without an inert gas atmosphere. The color of the dosage form produced according to the invention from the starting composition and of the dosage form produced by way of comparison i...
process embodiment 3
[0201] In this process variant for the production of the dosage form according to the invention energy is applied to a mixture of the components by means of ultrasonication.
[0202] First of all a homogeneous mixture of at least component (A) and component (C) (=binder) is produced. Further auxiliary substances, such as for example fillers, plasticisers, slip agents or dyes, may also be incorporated into this mixture. A low molecular weight polyethylene glycol is preferably used as plasticiser.
[0203] Mixing may be performed with the assistance of conventional mixers. Examples of suitable mixers are roll mixers, which are also known as tumbler, drum or rotary mixers, container mixers, barrel mixers (drum hoop mixers or tumbling mixers) or shaking mixers, shear mixers, compulsory mixers, plough bar mixers, planetary kneader-mixers, Z kneaders, sigma kneaders, fluid mixers or high-intensity mixers.
[0204] Selection of the suitable mixer is determined inter alia by the flowability and c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com