Optimized liquid-phase oxidation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
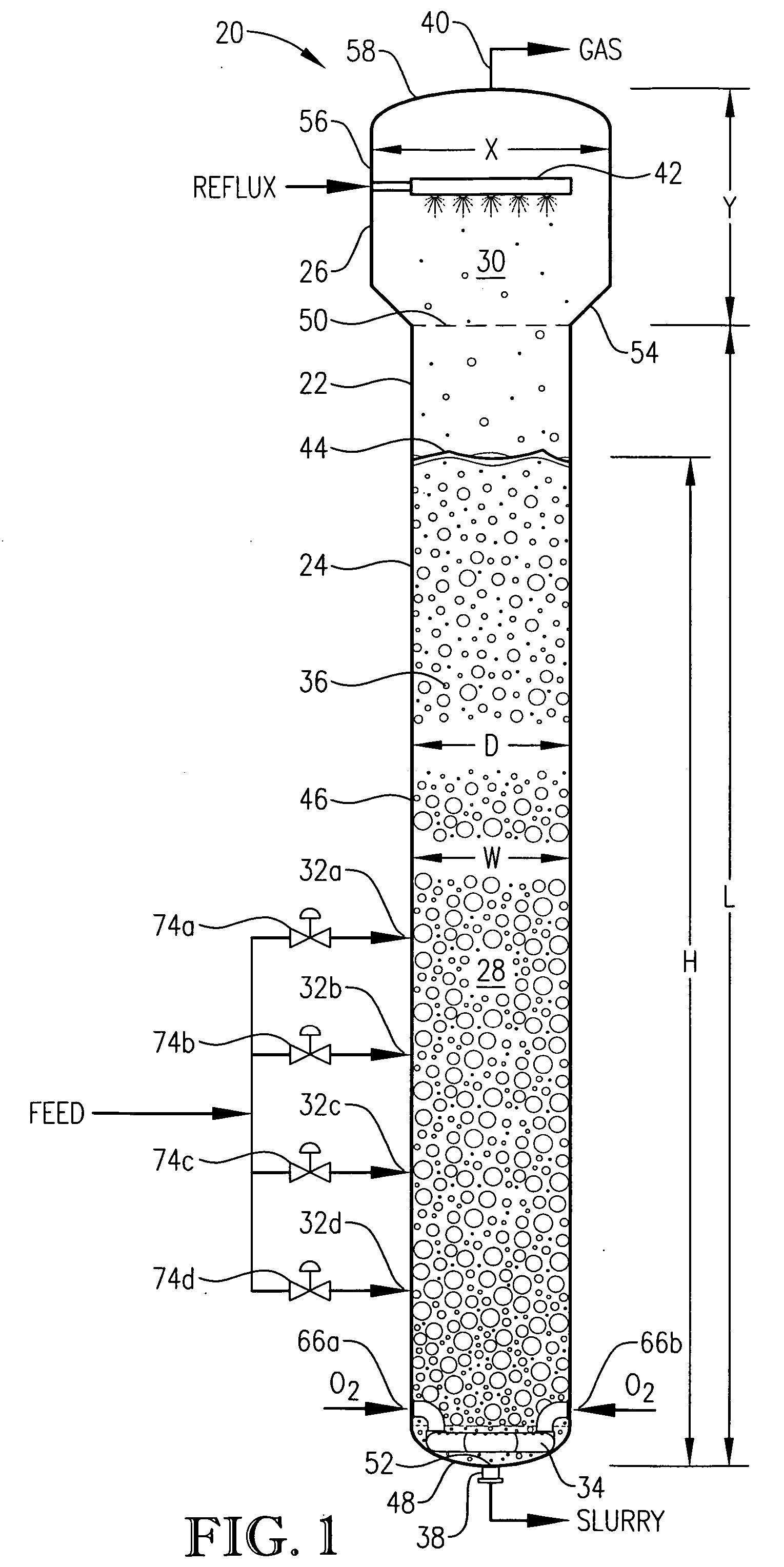
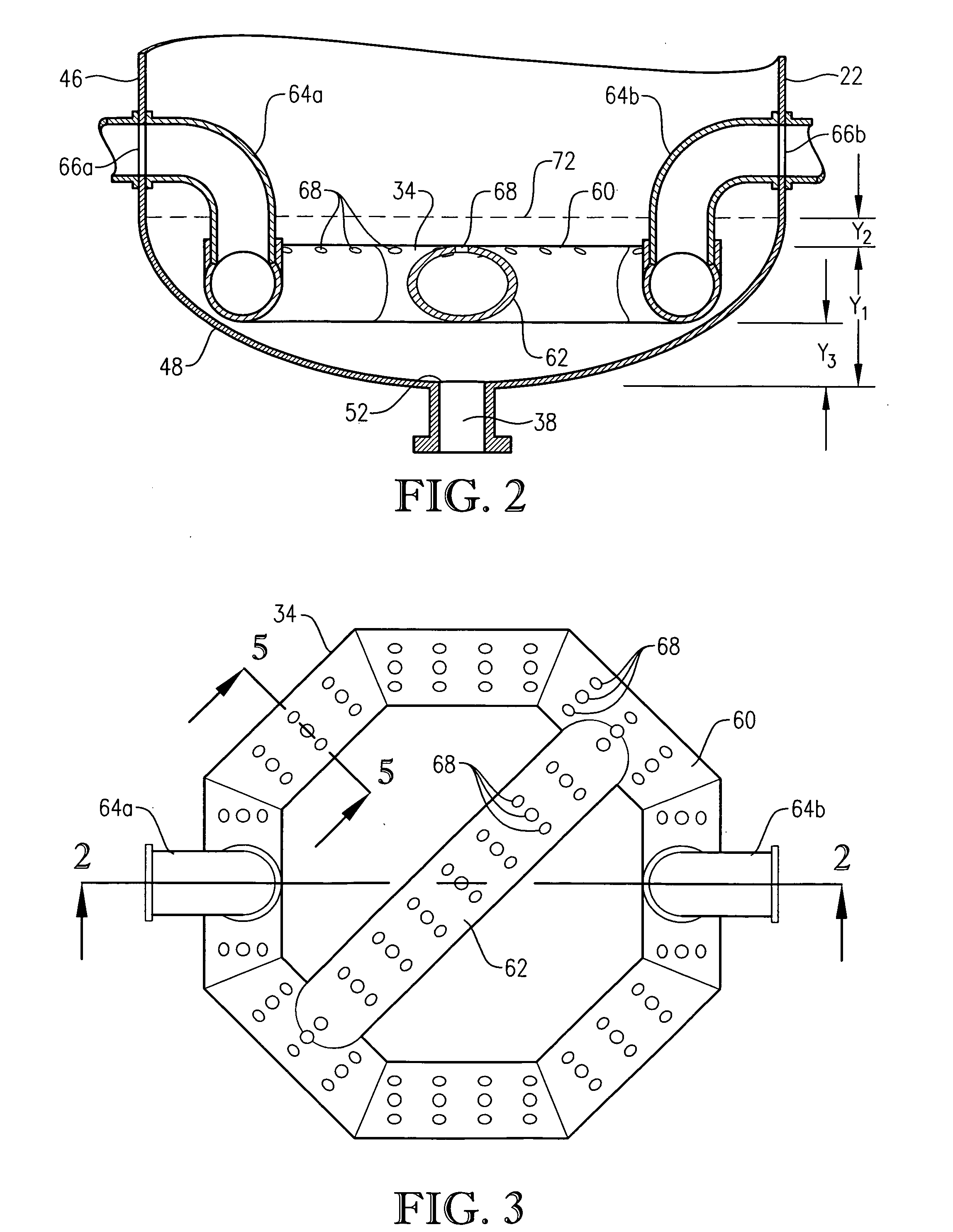
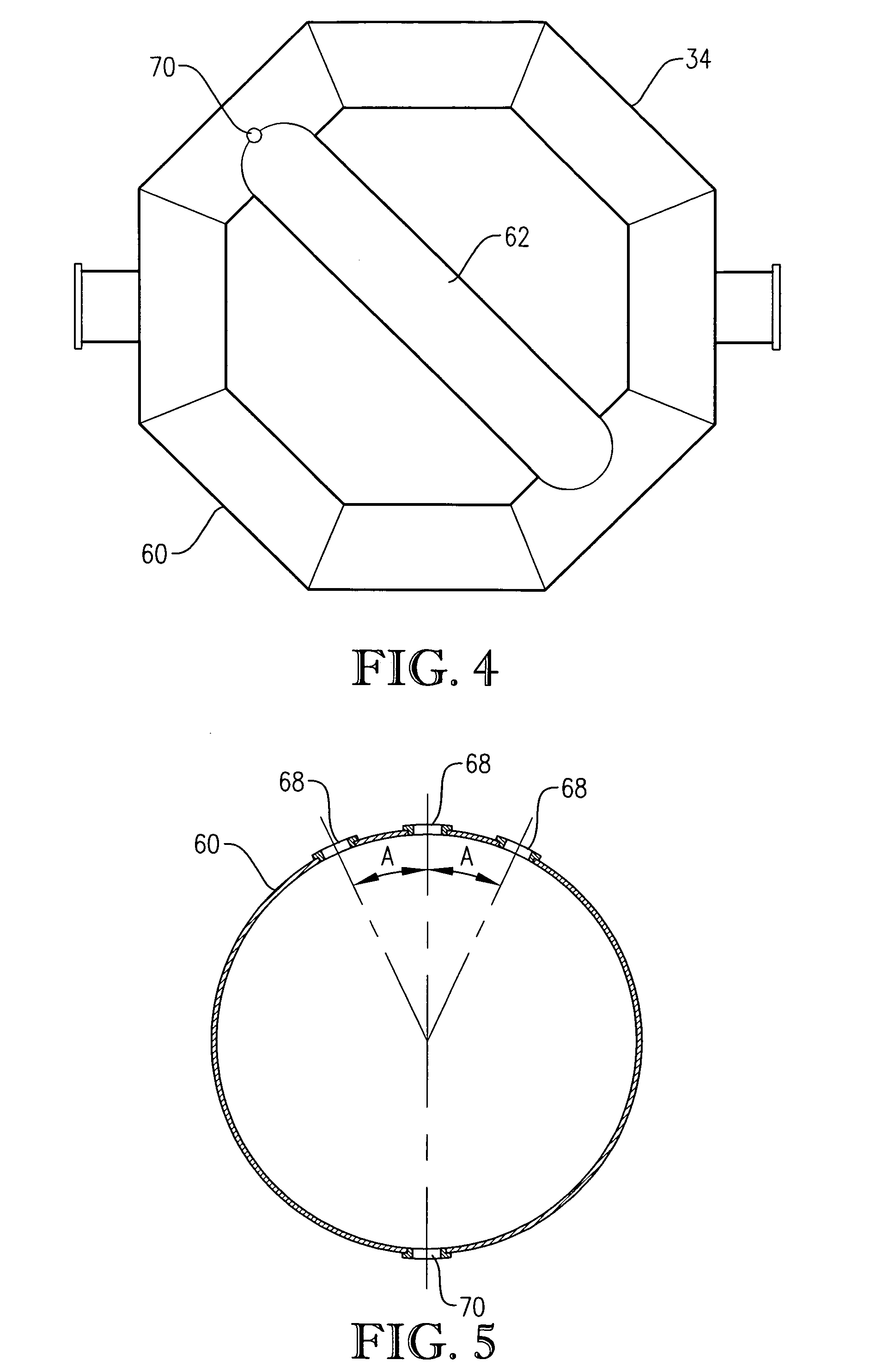
[0282] Examples 1-4 pertain to pilot-scale oxidations of para-xylene to terephthalic acid conducted in a pilot-scale system assembled around a mechanically agitated, hot-oil jacketed, 2-gallon, titanium reaction vessel. The gas dispersion type agitator within the reaction vessel was rotated at about 1,500 revolutions per minute (rpm), and the power draw of the agitator was about 220 watts. The pilot-scale system was equipped with means to control the pressure and temperature within the reaction vessel and to control the gas and liquid flow rates entering the reaction vessel. The feed of para-xylene was provided via a syringe pump at an effectively steady rate of about 0.28 kilograms per hour. Catalyst feed solution was pumped from a catalyst feed tank into the reaction vessel at an effectively steady rate of about 3.2 kilograms per hour. Both para-xylene and catalyst feed solution were released into the reaction medium through a dip tube ending below the level of aerated slurry with...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com