Compositions Of Bakuchiol And Methods Of Making The Same
a technology of bakuchiol and bakuchiol, which is applied in the field of bakuchiol compositions, can solve the problems of psoralens presence, number of limitations associated with the use of this compound, and the association of health risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Method for the Quantification of Bakuchiol, Psoralen and Isopsoralen by HPLC
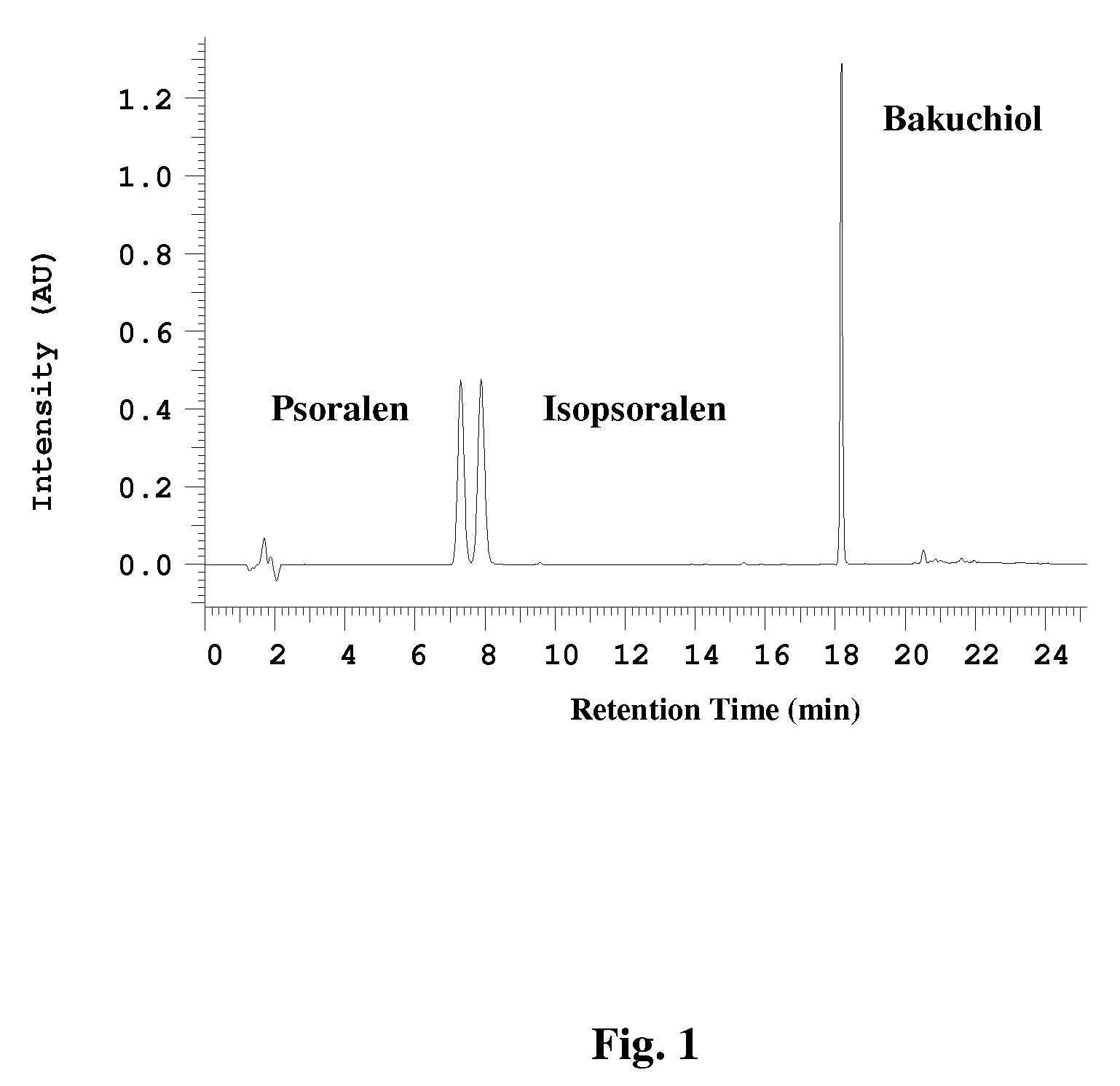
[0093] The amount of bakuchiol, psoralen and isopsoralen in the extracts, fractions, and the novel composition generated as described below was quantified by a high pressure liquid chromatography (HPLC) using a PhotoDiode Array detector (HPLC / PDA) and a Luna Phenyl-hexyl column (250 mm×4.6 mm). The targeted compounds were eluted from the column using an acetonitrile (ACN) water gradient from 36% to 100% ACN over a period of 12 minutes, followed by 100% ACN for three minutes. The detailed HPLC conditions used are set forth in Table 1. A chromatogram of the HPLC separation is depicted in FIG. 1. The targeted compounds were identified and quantified based on retention time and UV peak area using commercially available pure bakuchiol, psoralen and isopsoralen as quantification standards. The retention times for the bakuchiol, psoralen and isopsoralen were 18.19 minutes, 7.33 minutes and 7.95 minutes, respective...
example 2
General Methods for the Extraction of Bakuchiol from Psoralea Plants
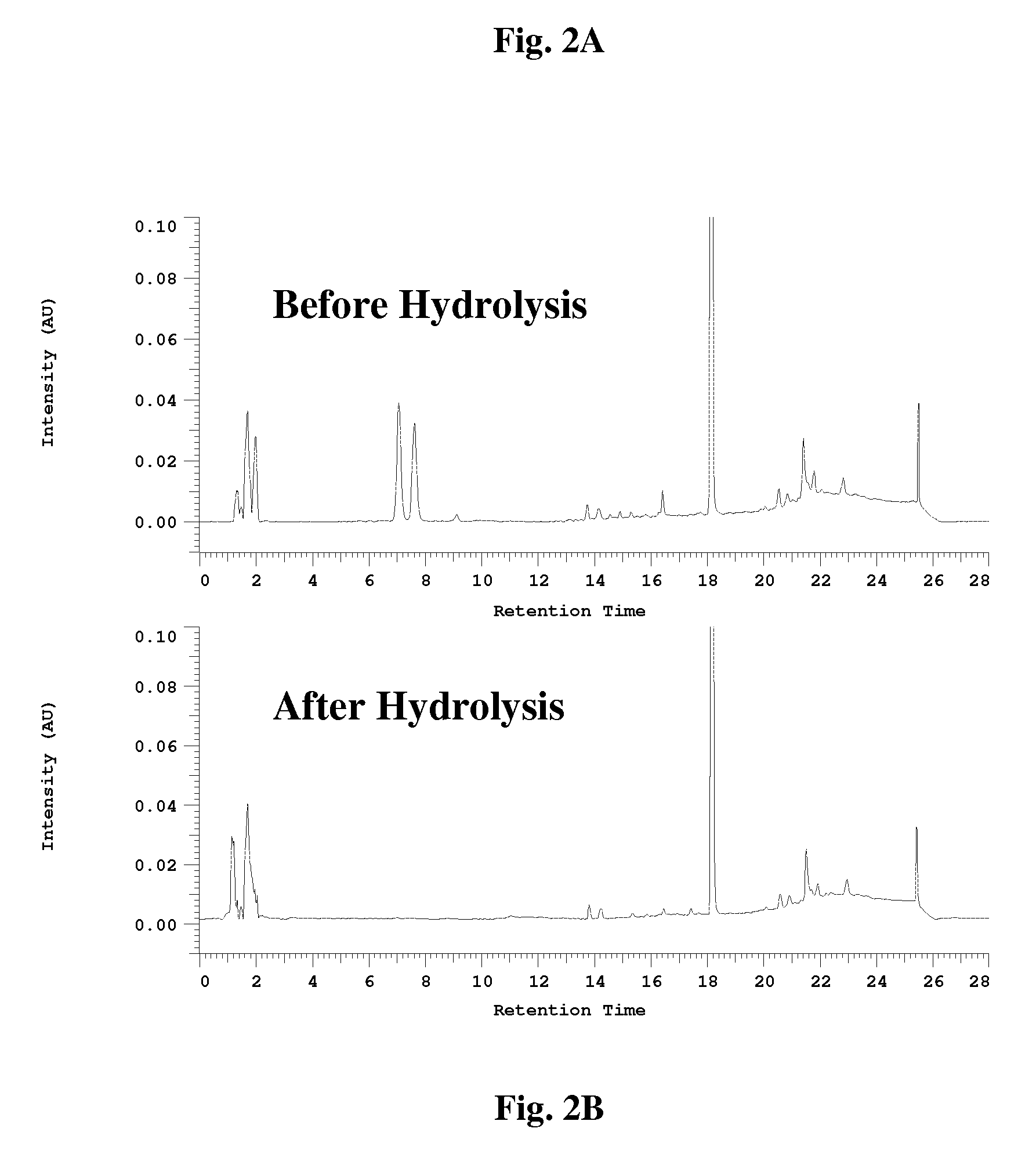
[0094] To a flask was added solvent (100 mL) and Psoralea corylifolia seed powder (10 g) and the mixture was shaken on a wrist shaker at room temperature for one hour. The mixture was then passed through a filter and the filtrate collected. The extraction process was repeated one more time with fresh solvent, the filtrates were combined, the solvent removed on a rotoevaporator and the residue was dried under high vacuum.
[0095] To a flask was added solvent (50 mL) and Psoralea corylifolia seed powder (10 g) and the mixture was refluxed for 40 min. The solution was then filtered and the extraction process was repeated two more times with fresh solvent. The filtrates were combined and the solvent was evaporated to obtain a dried extract.
[0096] Following above extraction methods, sample plant material was extracted with the following solvents: dichloromethane (DCM), EtOAc, acetone, MeOH, petrole...
example 3
Large Scale Extraction of Bakuchiol from Psoralea Plants
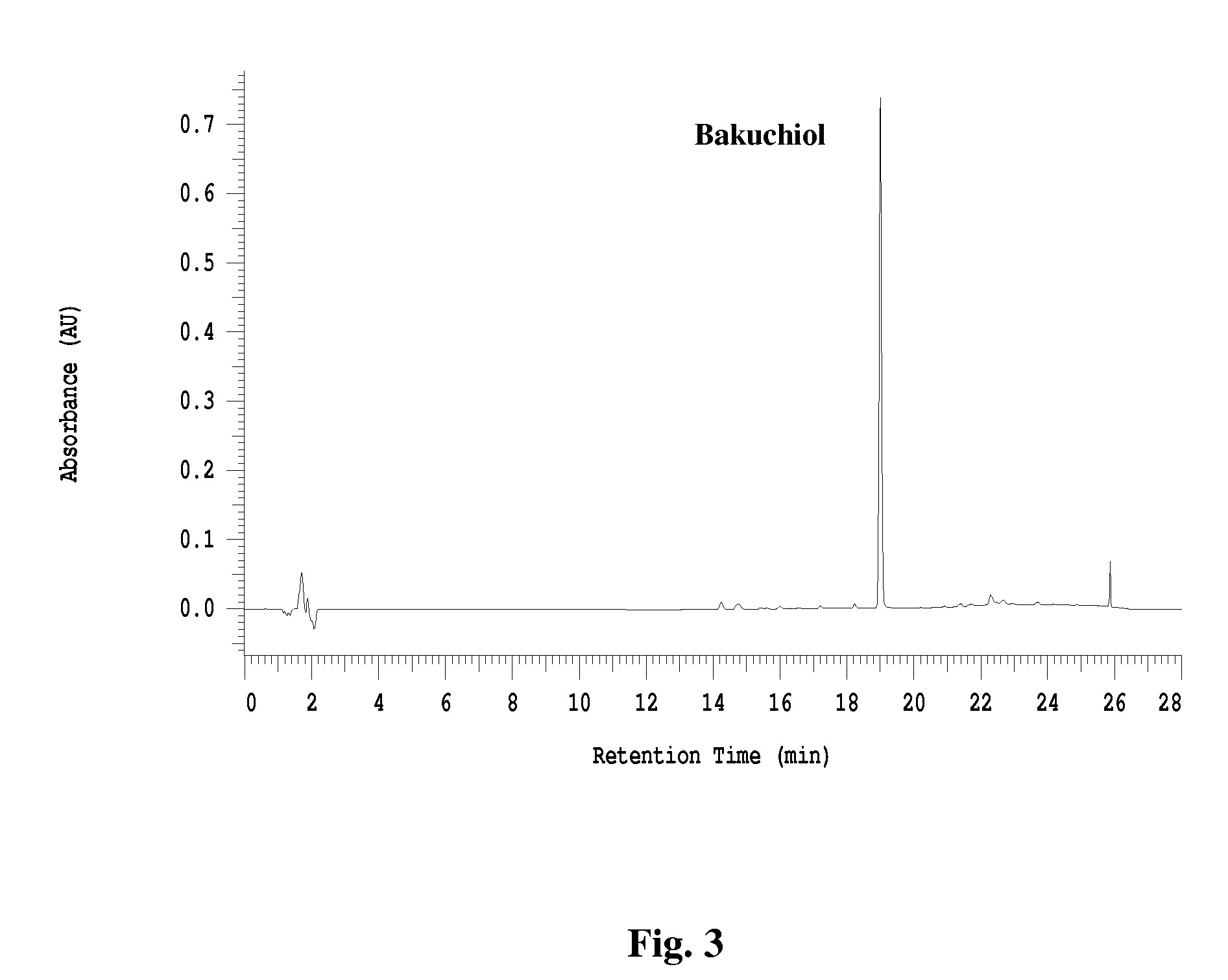
[0097] Seed powder of Psoralea corylifolia (2 kg) and 9 liter of petroleum (pet.) ether (BP 60-90° C.) were rotated in a 20 L flask on a rotoevaporator at 70° C. in a water bath for 1 hour. The solution was then decanted into a separate container and the solvent was removed under vacuum. Fresh solvent was added into the biomass and the extraction process was repeated three more times. The extracts were combined and evaporated to yield 335 g of a crude extract (MH-258-01-01) having 21% bakuchiol and 3% psoralen / isopsoralen by weight.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight percent | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com