Metal fine particles, composition containing the same, and production method for producing metal fine particles
a technology of metal fine particles and compositions, applied in the field can solve the problems of reducing the absorption capacity binders, and reliability, and achieve the effects of reducing the absorption capacity, reducing the stability of metal fine particles, and increasing the cost of metal fine particles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production Method for Gold Fine Particles
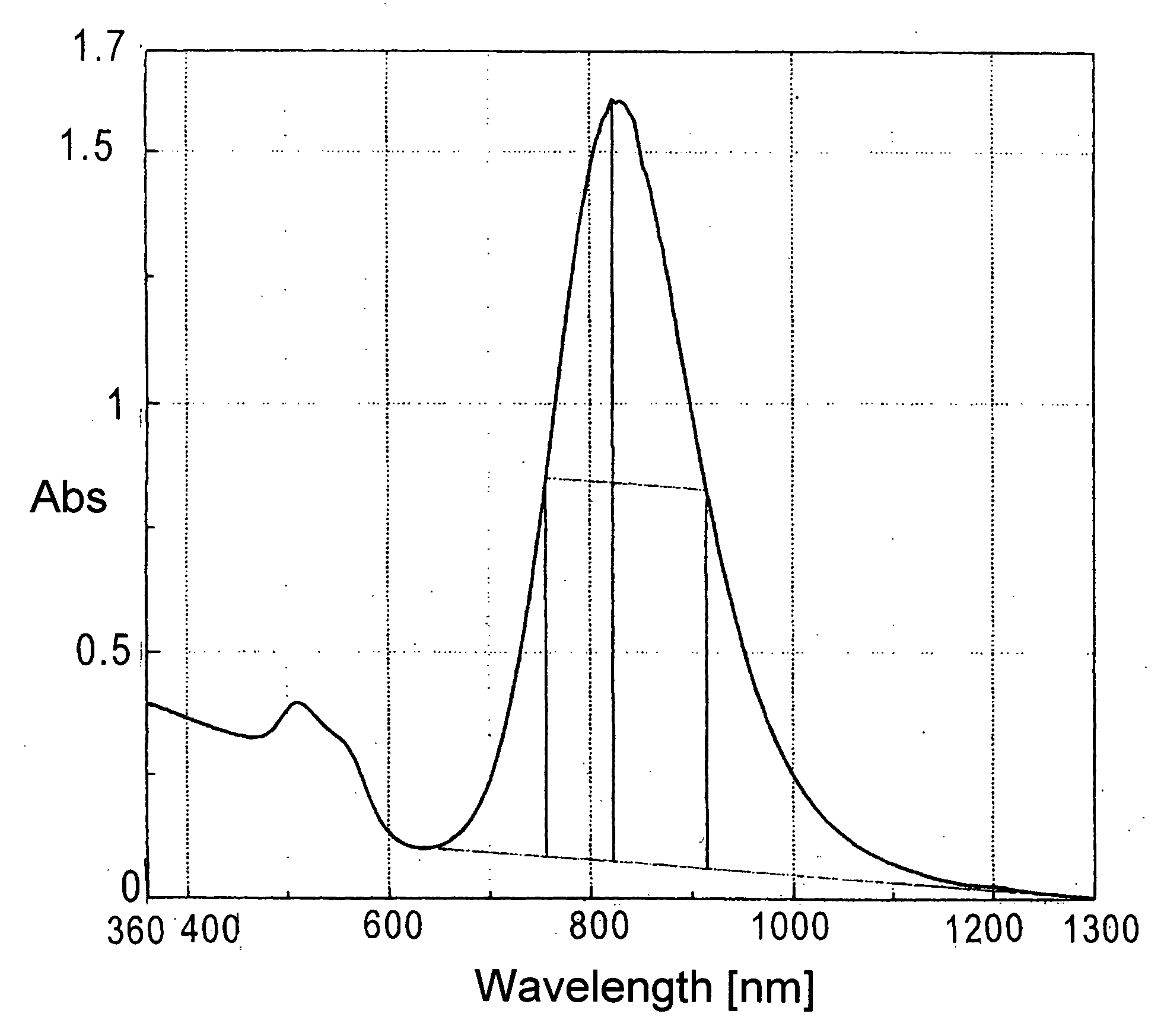
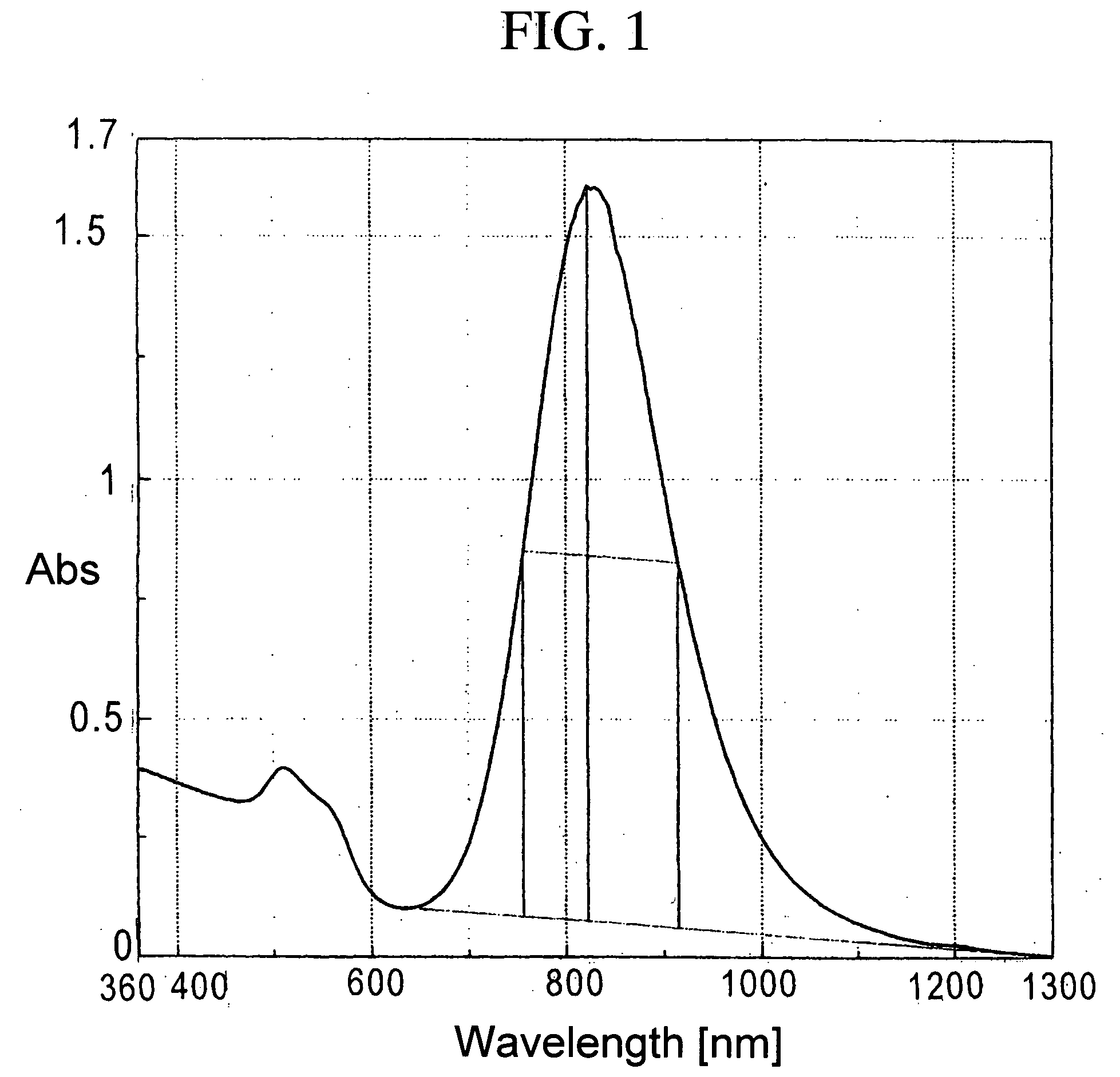
[0100] To 50 ml of 0.50 mol / L-CTAB (hexadecyltrimethylammonium bromide) aqueous solution, 5 ml of 24 mmol / L-chloroauric acid aqueous solution, 1 ml of acetone, 1 ml of cyclohexane, 1 ml of cyclohexanone, and 5 ml of 10 mmol / L-silver nitrate aqueous solution were added to produce the reaction solution. To the reaction solution, 5 ml of 40 mmol / L-ascorbic acid (AS) aqueous solution was added to initiate chemical reduction. Just after the AS aqueous solution was added, the color of the reaction solution changed from orange to transparent and colorless. The transparent and colorless solution was put into a 100 ml-beaker, and ultraviolet light generated in a UV irradiation device (high-pressure mercury lamp) was irradiated directly onto the synthesis solution from the upper part of the beaker for five minutes. After irradiation, the synthesis solution was left to rest for one hour, and transferred to a storage vessel. Then the solution was ten-t...
example 2
Gold Fine Particles Surface-Treated with a Dispersing Agent
[0102] 0.1 g of the dispersing agent (Solsperse 24000SC; marketed by Avecia Ltd.) was dissolved in 10 g of toluene. To the toluene solution containing the dispersing agent, 50 g of the aqueous dispersing solution of the gold nano-rods (the average length in the short axis: 10 nm; the average length in the long axis: 42 nm, and the aspect ratio: 4.2) synthesized in Example 1, was added, and they were aggregated for ten minutes using an aggregator (revolution speed: 300 rpm). To the obtained solution, 30 g of ethanol was added, and this was left to rest for twenty-four hours. The solubility of CTAB increased by adding ethanol, and CTAB absorbed in the surface of the gold nano-rods was desorpted. Then, nitrogen portions of the dispersing agent were absorbed in gold nano-rods and they were replaced with CTAB, and the surface treatment was performed.
[0103] The mixture, which was left to rest, was separated into a transparent a...
example 3
Gold Fine Particle Composition and Film
[0105] The coating was obtained by mixing 5 g of the gold nano-rods concentrated solution obtained in Example 2 in 20 g of a mixture containing a radical polymerizable urethane oligomer and a radical polymerization initiator. The obtained coating did not change color or generate precipitations, and was stable even though it was left under conditions in which light was blocked and at room temperature for three months or longer.
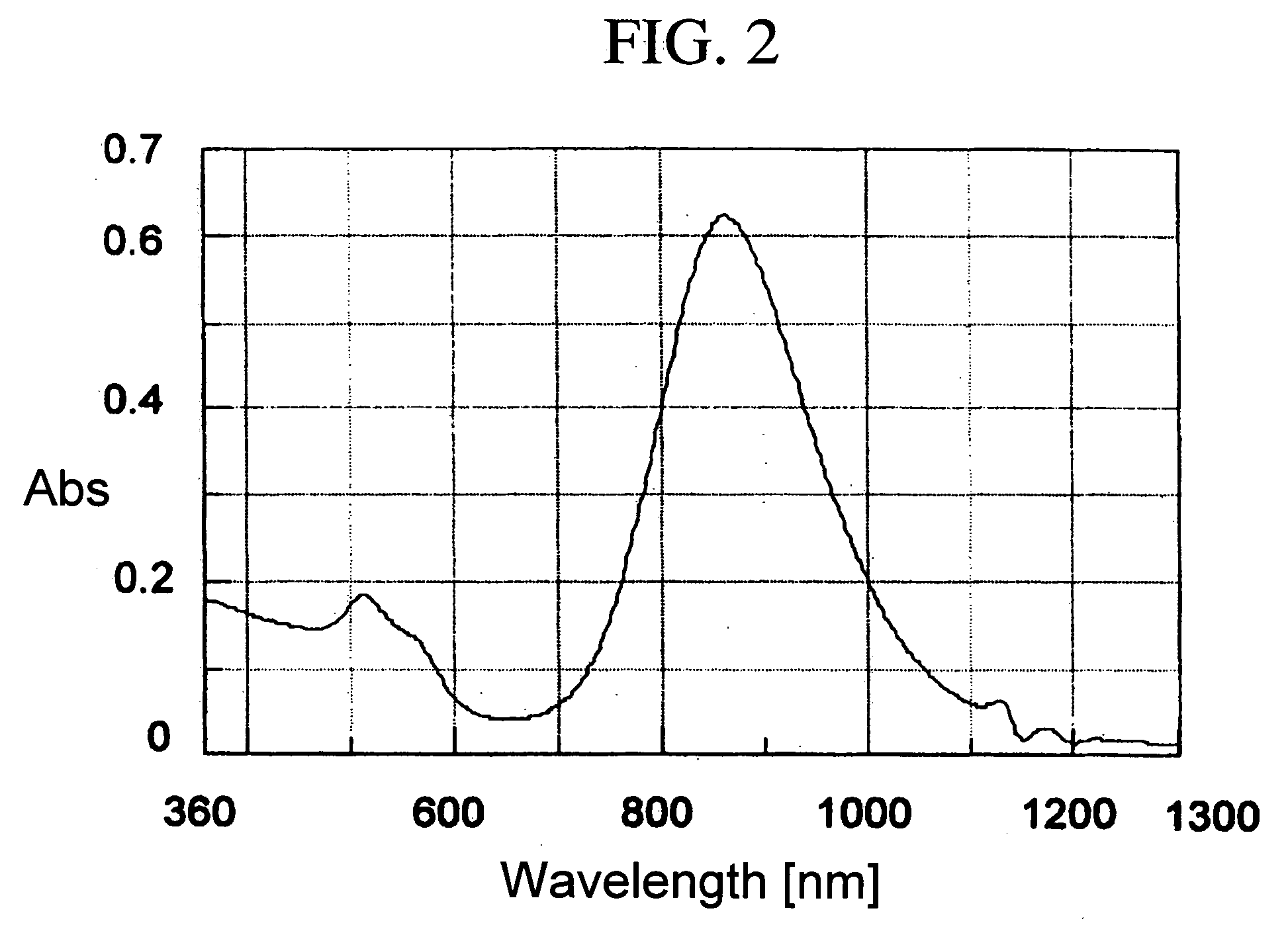
[0106] The coating was coated on the glass plate (gold fine particle content: 1% by weight, and dried film thickness: 10 μm), and the transmitted spectrum was measured. The results are shown in FIG. 3. As shown in FIG. 3, the transmissivity around the wavelength (870 nm) which corresponds to the peak position of the maximum absorption wavelength shown in FIG. 2 was the lowest. Thereby, it was confirmed that the specific wavelength was absorbed by the gold nano-rods.
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption wavelength | aaaaa | aaaaa |
| half band width | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com