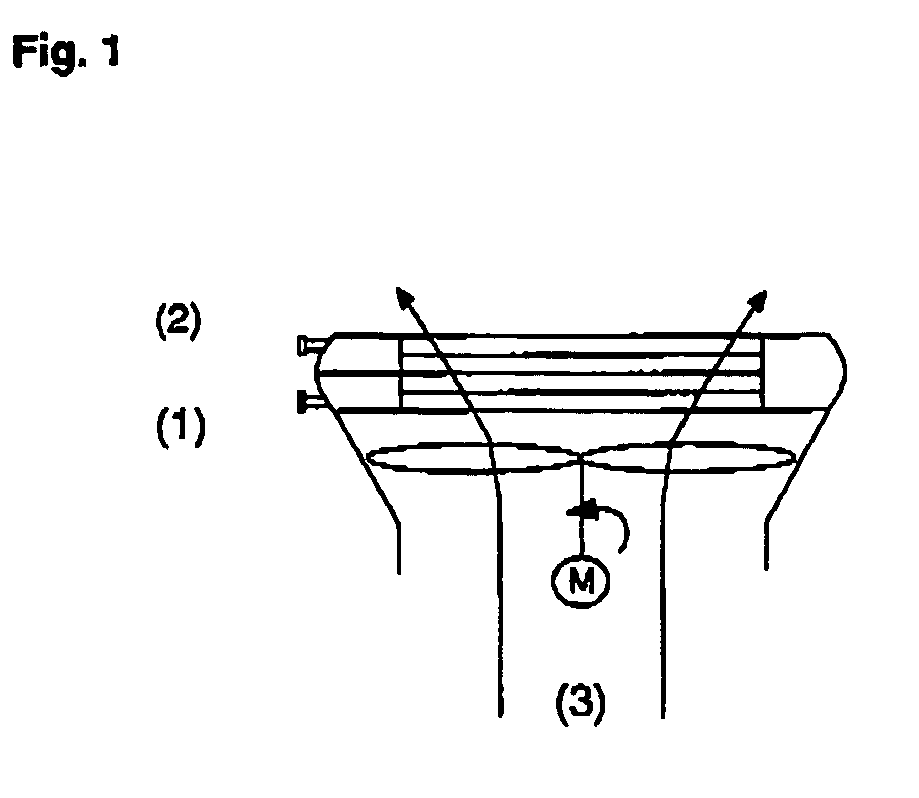
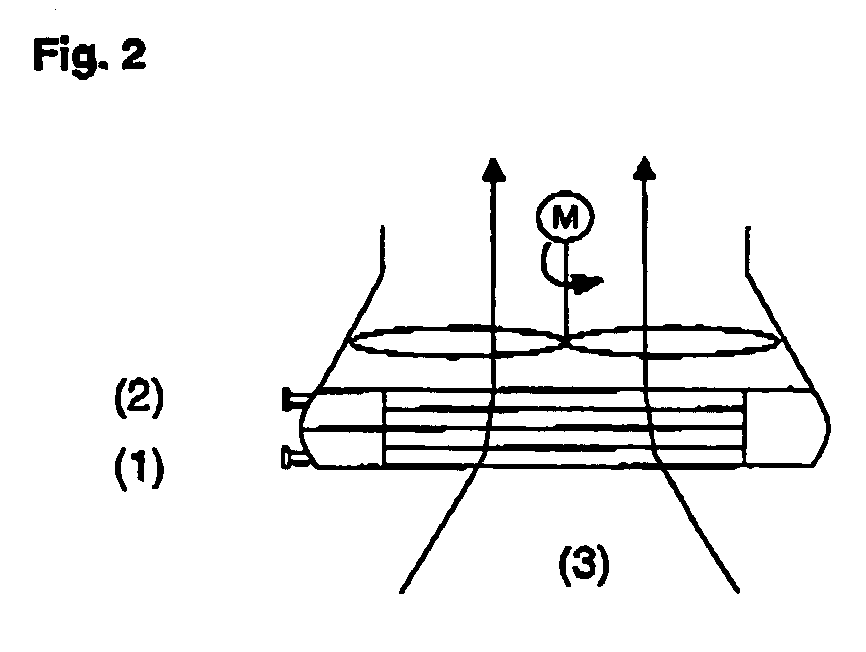
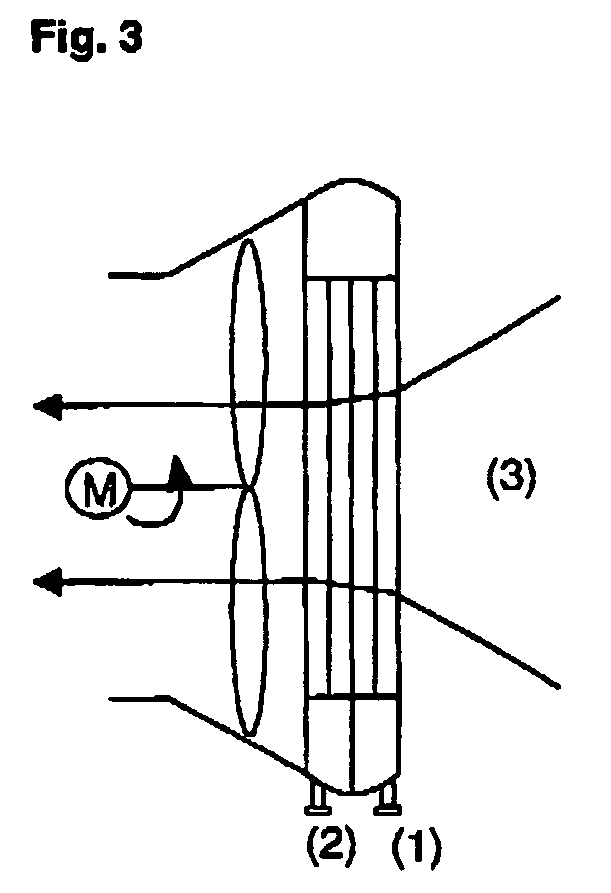
Process for producing propylene oxide
a propylene oxide and process technology, applied in the direction of organic chemistry, etc., can solve the problems of high cost, inability to meet the specific requirements of removing oxygen, and in the prior art description of catalysts, etc., to reduce the oxygen comprised
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of a Catalyst According to the Invention
[0297] As support, alpha-alumina spheres were used which are commercially available (Spheralite 512 G from Axens, France).
[0298] 225 g of these alumina spheres were impregnated with 86 ml of a solution of 0.3134 g of platinum nitrate having a platinum content of 57.52 wt.-%. After 2 h, the impreganted catalyst support was dried at 120° C. The dried catalyst was subsequently impregnated with 77 ml of a solution of 0.3427 g of tin (II) chloride dihydrate. The catalyst was then dried at 120° C. and calcined at 500° C. for 3 h. The thus obtained catalyst had the following composition:
alpha-alumina:99.84 wt.-%platinum: 0.08 wt.-%tin: 0.08 wt.-%
example 2
Epoxidation of Propene
[0299] A stream consisting of 54.5 g / h chemical grade propylene (98 wt.-%) was epoxidized with 74.7 g / h crude aqueous hydrogen peroxide (40 wt.-%) in the presence of a methanol stream (299 g / h) at a pressure of 20 bar. Epoxidation was carried out in the presence of 100 g TS-1 catalyst. The yield of propylene oxide, based in hydrogen peroxide, was 93.2% at a hydrogen peroxide converision of 99.8%.
[0300] Separation of the light components, methanol and water from the main reaction product was performed in a distillation tower having 40 trays. At a top pressure of 1.1 bar, a top stream of the distillation tower was obtained giving a stream (17.5 g / h) containing 83 wt.-% propane, 12 wt.-% propane, 0.6 wt.-% oxygen, 3.3 wt.-% methanol, and 1 wt.-% water. Propylene oxide, methanol and water were taken from the bottom of the distillation tower.
[0301] The repetition of the same experiment using polymer grade propylene resulted in a stream (15.9 g / h) containing 91.2...
example 3
Reaction of Mixture (GII) Obtained According to Example 2 Using the Catalyst According to Example 1
[0302] A stream obtained according to example 2 was compressed to a pressure of 16 bar. At this pressure, condensation at 40° C. was performed giving a liquid and a gaseous stream. The gaseous stream contained 2.8 vol.-% oxygen, 95.3 vol.-5 propene, 0.6 vol.-% propane, 0.7 vol.-% methanol and 0.5 vol.-% water.
[0303] This stream (238.5 Nl / h) was subjected to hydrogenation at a temperature of 300° C. and a pressure of 12 bar using a hydrogen stream (22.7 Nl / h) in a fixed-bed reactor containing 100 g catalyst according to example 1.
[0304] An oxygen conversion of at least 99.6% was achieved, corresponding to an oxygen content of the reactor effluent of at most 100 ppm. Hydrogen conversion was above 97.5 5, propene conversion was 5.4%. The yield with respect to propane was 5.3%, the yield with respect to COx compounds 0.1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pressure | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com