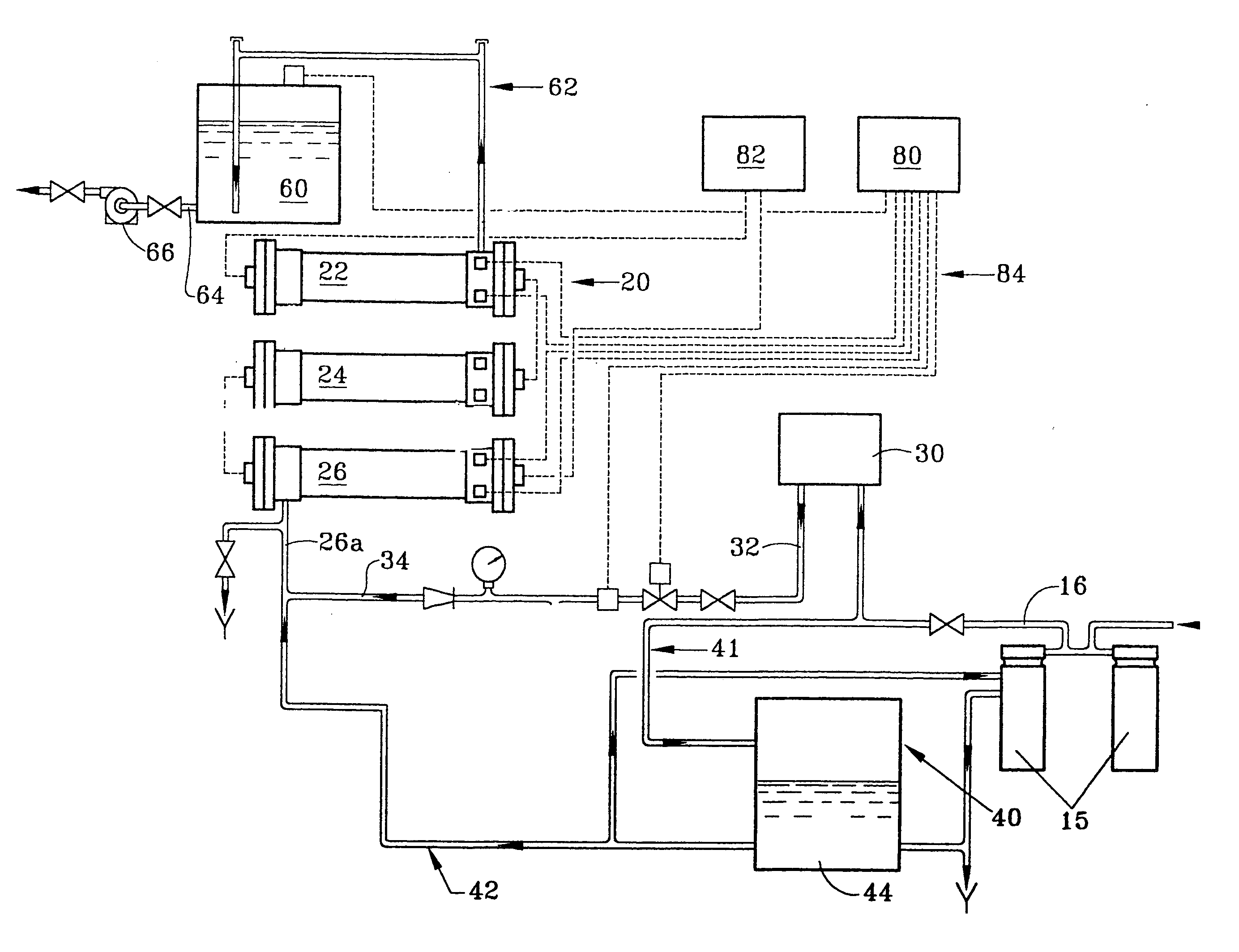
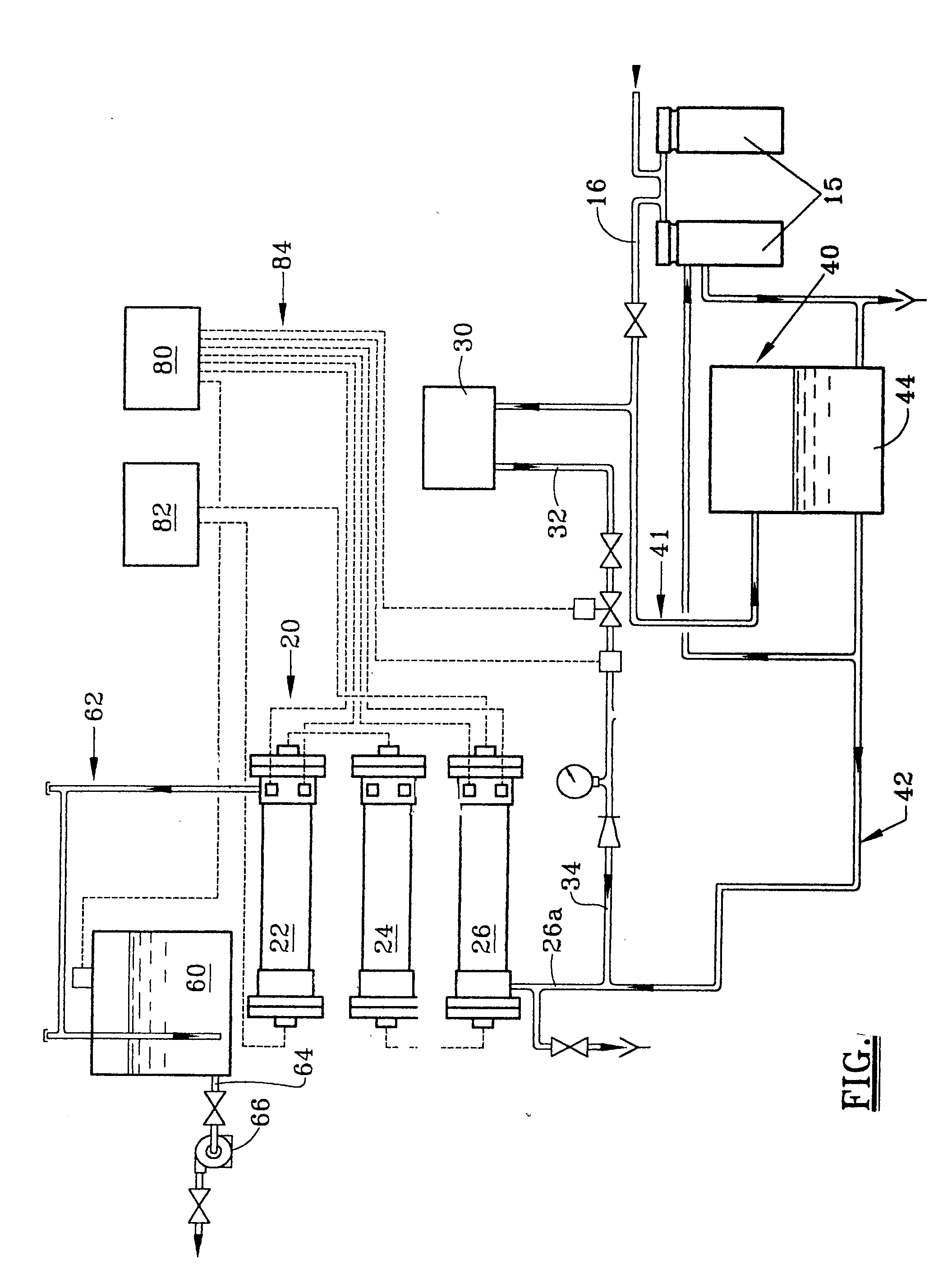
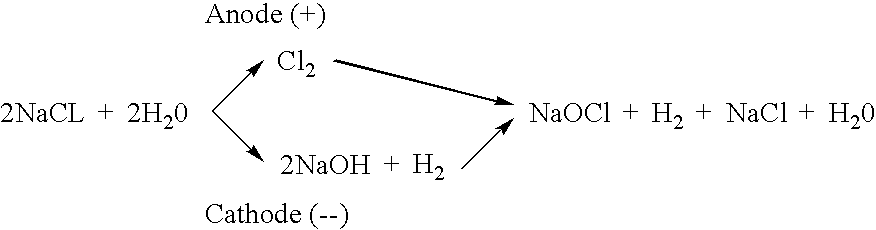Process for producing hypochlorite
a technology of hypochlorite and process, which is applied in the direction of multiple component coating, electrolysis components, electrolysis coating, etc., can solve the problems of affecting the amount of hypochlorite produced, affecting the economics of hypochlorite production, and high cost of commercial hypochlorite transportation, so as to reduce salt and power consumption, improve current efficiency, and improve the effect of efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
— example 1
Test Procedure—Example 1
[0048] A chloride brine was fed into a test electrolytic cell at a brine feed rate equivalent to 12-16 gallons per pound of product produced. This cell had no technological improvements such as coated cathodes or chilled solutions. The concentration of chloride salt within the brine solution was maintained between 28-30 grams per liter. The liquid flow rate was maintained so as to maintain the same exit hypochlorite concentration if the cell efficiency remained constant. Temperatures were ambient. In this way, any improvement in future tests for hypochlorite concentration is related to improved cell efficiency. A baseline performance profile with the above operating conditions and titanium cathodes was obtained.
Process for Producing HypochioriteExample ITestPowerCell InletCell OutletNaClEff. GramsNaoClGPLCurrent#Cons. DCTempTempGPLNaCl / NaoClGPLTheo.Eff. %12.22045333.649.0814.363.522.022045303.219.3613.768.431.992345303.189.4313.669.342.0820453...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com