Use of ramoplanin to treat diseases associated with the use of antibiotics
a technology of ramoplanin and ramoplanin, which is applied in the direction of antibacterial agents, drug compositions, peptide/protein ingredients, etc., can solve the problems of clindamycin patients being highly susceptible to cdad, allergic reactions, intolerances, and so as to inhibit the relapse of antibiotic-associated diarrhea
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Efficacy of Ramoplanin in the Hamster Model of C. difficile Associated Colitis
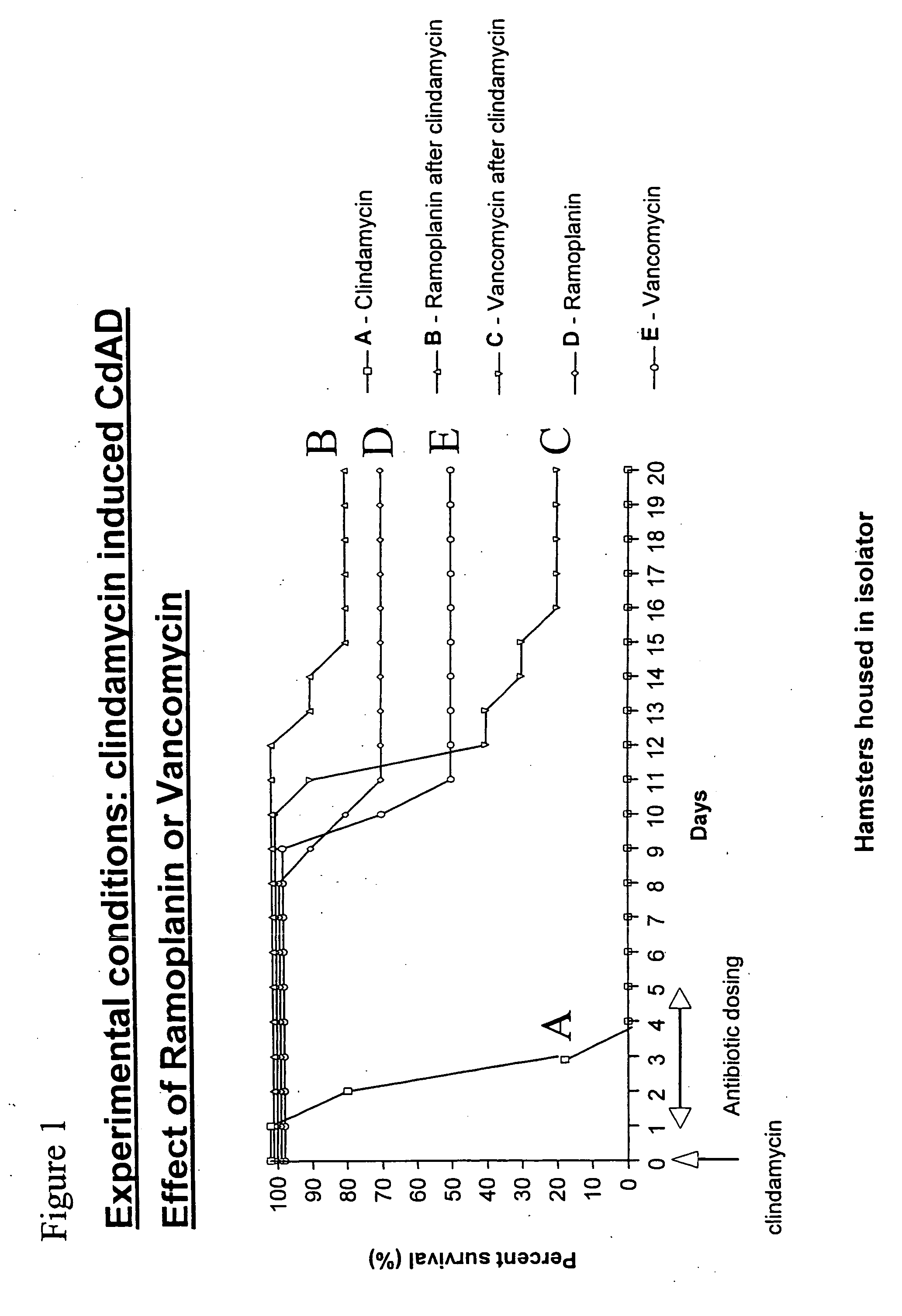
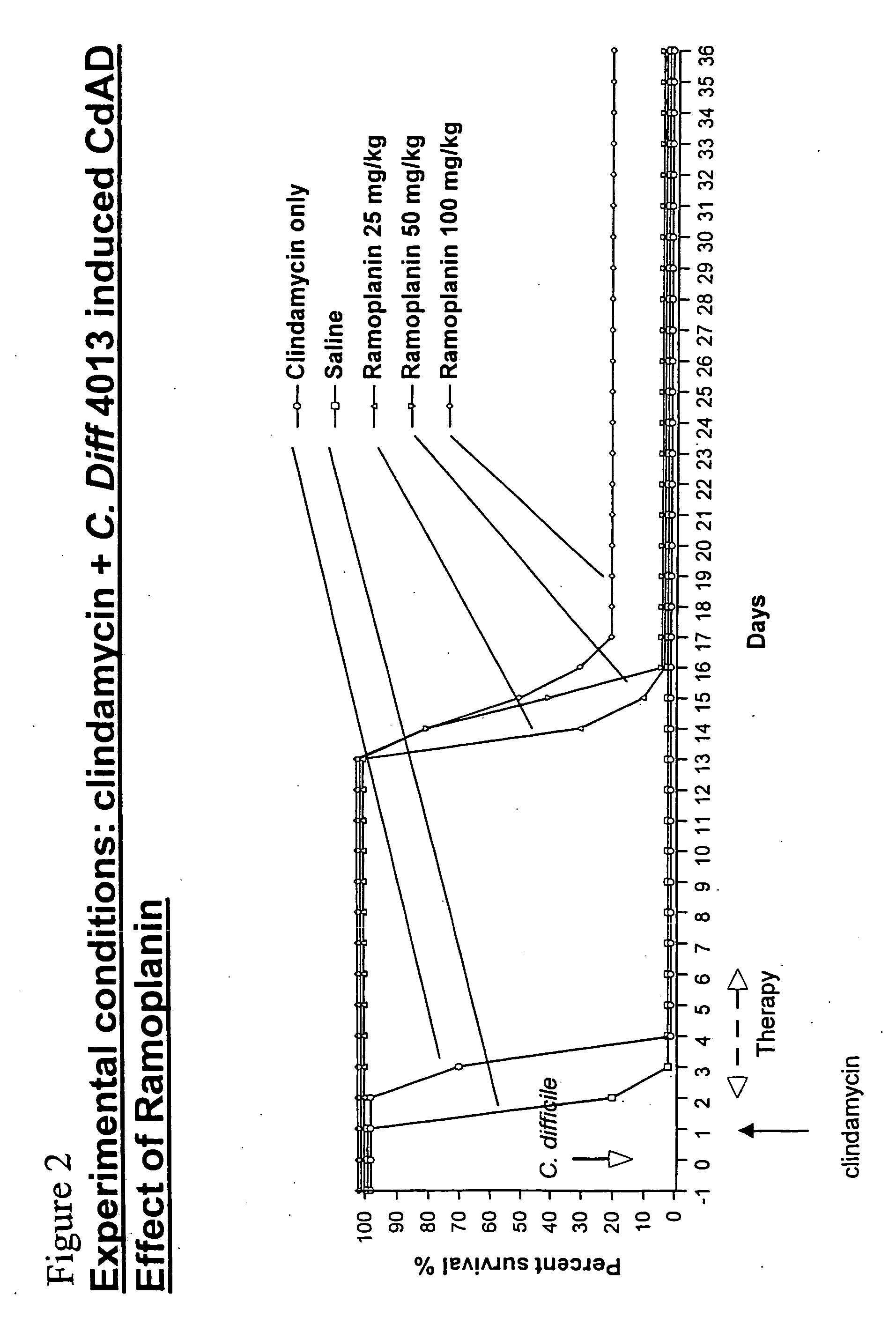
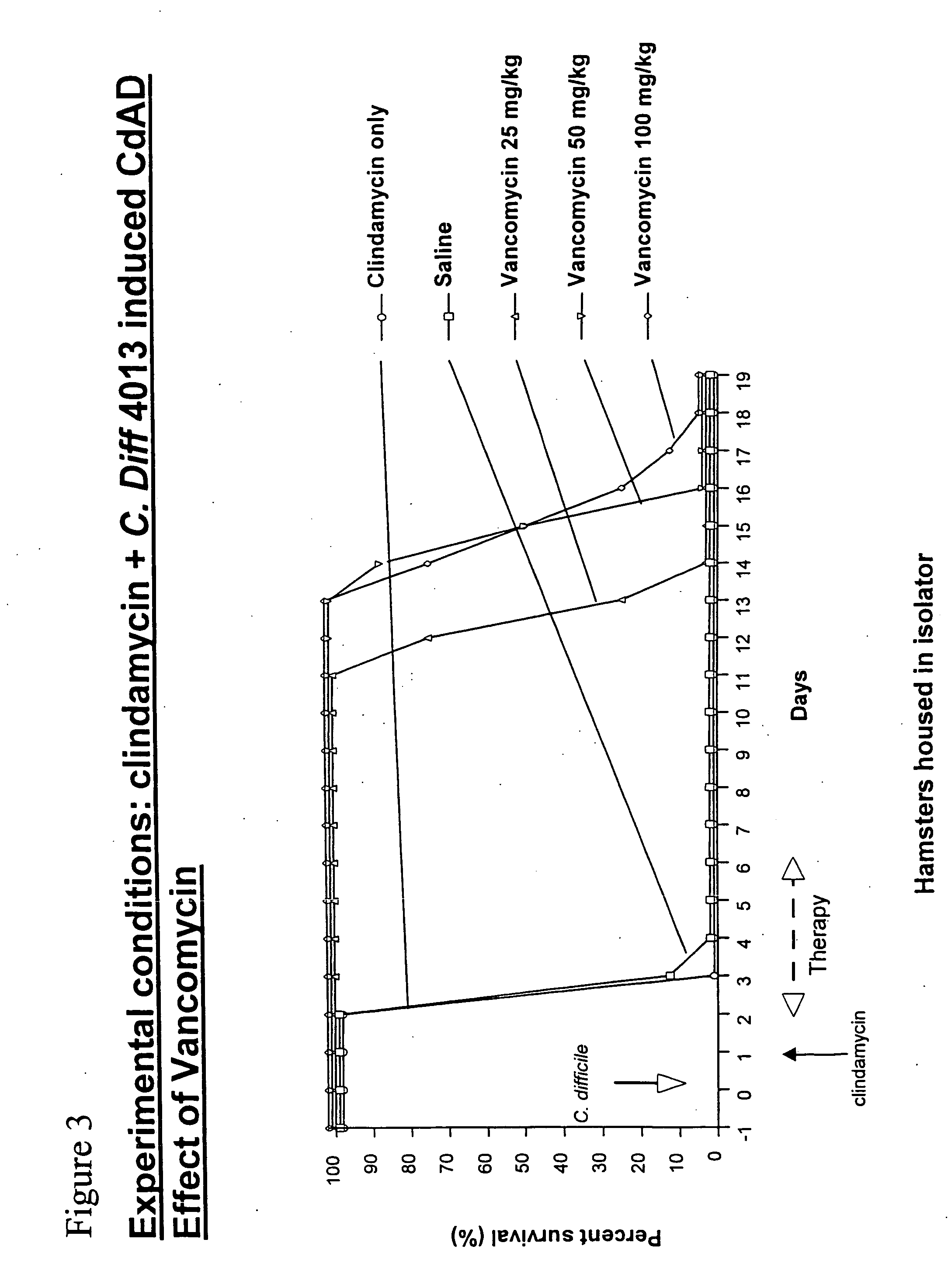
[0068] To evaluate the in vivo efficacy of ramoplanin in the treatment of C. difficile-associated colitis, ramoplanin was tested in a hamster model of clindamycin (CL)-induced colitis in comparison with both vancomycin and metronidazole. Animals were treated with a single subcutaneous (s.c.) injection of 100 mg / kg clindamycin, and after 24 hours received oral ramoplanin or vancomycyin at 50 mg / kg / day for 5 days. Animals were observed daily for the presence or absence of diarrhea. Necropsies were performed on some animals that died during the experiment, and cecal contents were assayed for C. difficile toxin A. Hamsters were monitored for 20 days, and the cumulative mortality during this period was recorded (FIG. 1). Clindamycin alone rapidly induced a fatal enterocolitis with 100% mortality within 4 days. Autopsy revealed hemorrhagic ceca and watery stools. C. difficile toxin A was always detected in thes...
example 2
Oral Administration of Ramoplanin to Humans
[0072] As is described in detail below, single oral doses (up to 1000 mg) and multiple oral doses (200, 400, or 800 mg BID for 10 days) of ramoplanin have been administered to healthy male volunteers. Both bioassay and HPLC-based assays to assess the absorption, distribution, metabolism, and excretion were utilized in these studies. Ramoplanin was not detected in serum / plasma or urine by either method, indicating that very little, if any, is absorbed. Treatment with oral ramoplanin at all doses was efficacious in reducing the Gram-positive colony counts in feces to undetectable levels during the 10-day regimen. Ramoplanin was not effective against Gram-negative flora
[0073] Single Dose Study in Healthy Male Volunteers
[0074] The absorption, tolerability, and recovery of ramoplanin following single dose oral administration were investigated in male volunteers. Ramoplanin was administered as an aqueous solution at a dose of 100, 200, 500, or...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| resistance | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com