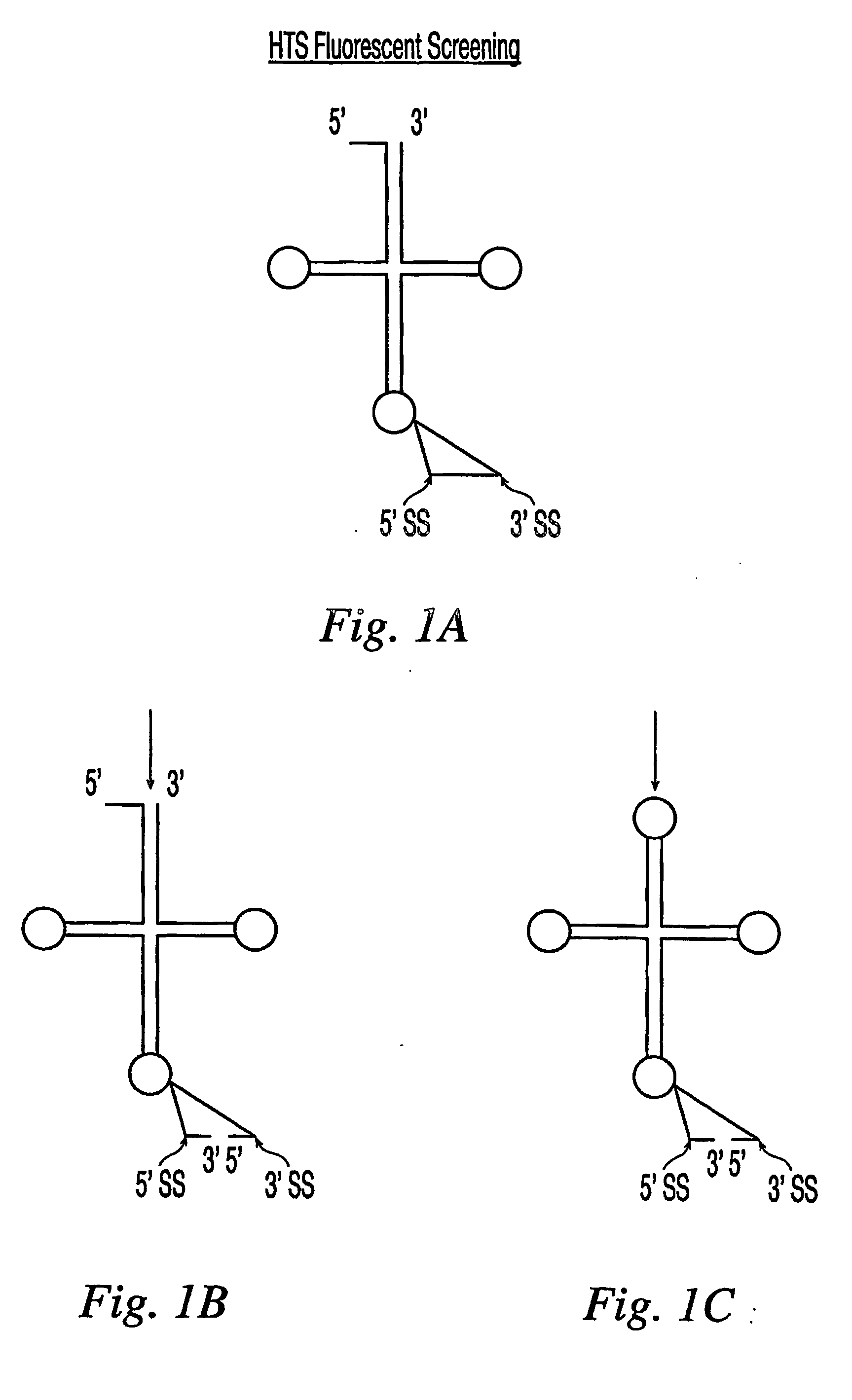
[0020] Fluoroscent
resonance energy transfer (“FRET”) assays may be used to identify a compound that modulates the activity of an animalia tRNA splicing endonuclease. The FRET assays may be conducted utilizing labeled subunits of an animalia tRNA splicing endonuclease or labeled substrates for an animalia tRNA splicing endonuclease. The FRET
cell-based assays may be conducted by microinjecting or transfecting a substrate for an animalia tRNA splicing endonuclease into an animalia
cell and contacting the
cell with a compound, wherein the substrate is labeled at the 5′ end with a
fluorophore and labeled at the 3′ end with a quencher, or, alternatively, the substrate is labeled at the 5′ end with a quencher and labeled at the 3′ end with a
fluorophore, and measuring the
fluorescence of the substrate by, e.g.,
fluorescence microscopy or a
fluorescence emission
detector such as a Viewlux or Analyst. The endogenous tRNA splicing endonuclease will cleave the substrate and result in the production of a detectable fluorescent
signal. A compound that inhibits or reduces the activity of the endogenous tRNA splicing endonuclease will inhibit or reduce the cleavage of the substrate and thus, inhibit or reduce the production of a detectable fluorescent
signal. A compound that enhances the activity of the endogenous endonuclease will enhance the cleavage of the substrate and thus, increase the production of a detectable fluoroscent
signal. Alternatively, the FRET cell-based assays may be conducted by microinjecting or transfecting a substrate for an animalia tRNA splicing endonuclease into a cell and contacting the cell with a compound, wherein the substrate is labeled at the 5′ end with a fluorescent donor
moiety and labeled at the 3′ end with a fluorescent
acceptor moiety, or alternatively, the substrate is labeled at the 5′ end with a fluorescent
acceptor moiety and labeled at the 3′ end with a fluoroscent donor moiety, and measuring the fluorescence of the substrate by, e.g., fluoresence
microscopy or a fluorescence emission
detector such as a Viewlux or Analyst. The endogenous tRNA splicing endonuclease will cleave the substrate and result in a decrease in the fluorescence emission by the fluorescent donor moiety and fluorescent
acceptor moiety at the
wavelength of the fluorescent donor moiety. A compound that inhibits or reduces the activity of the endogenous tRNA splicing endonuclease will inhibit or reduce cleavage of the substrate and thus, increase the fluorescence emission of the fluorescent acceptor moiety at the
wavelength of the fluorescent donor moiety. A compound that enhances the activity of the endogenous tRNA splicing endonuclease will enhance the cleavage of the substrate and thus, reduce the fluorescence emission of the fluorescent acceptor moiety at the
wavelength of the fluorescent donor moiety.
[0024] The FRET cell-free-based assays may be conducted by contacting a substrate for an animalia tRNA splicing endonuclease with an animalia cell-free extract (preferably, a tRNA splicing endonuclease extract) or a purified animalia tRNA splicing endonuclease and a compound, wherein the substrate is labeled at the 5′ end with a
fluorophore and labeled at the 3′ end with a quencher, or, alternatively, the substrate is labeled at the 5′ end with a quencher and labeled at the 3′ end with a fluorophore, and measuring the fluorescence of the substrate by, e.g., a fluorescence emission
detector such as a Viewlux or Analyst. The tRNA splicing endonuclease in the animalia cell-free extract or the purified animalia tRNA splicing endonuclease will cleave the substrate and result in the production of a detectable fluorescent signal. A compound that inhibits the activity of the animalia tRNA splicing endonuclease will inhibit or reduce the cleavage of the substrate and thus, inhibit or reduce the production of a detectable fluorescent signal. A compound that enhances the activity of the animalia tRNA splicing endonuclease will enhance the cleavage of the substrate and thus, increase the production of a detectable fluorescent signal. Alternatively, the FRET cell-free-based assays may be conducted by contacting a substrate for an animalia tRNA splicing endonuclease with an animalia cell-free extract or a purified animalia tRNA splicing endonuclease and a compound, wherein the substrate is labeled at the 5′ end with a fluorescent donor moiety and labeled at the 3′ end with a fluorescent acceptor moiety, or, alternatively, the substrate is labeled at the 5′ end with a fluorescent acceptor moiety and labeled at the 3′ end with a fluorescent donor moiety, and measuring the fluorescence of the substrate by, e.g., a fluorescence emission detector such as a Viewlux or Analyst. The tRNA splicing endonuclease in the animalia cell-free extract or the purified animalia tRNA splicing endonuclease will cleave the substrate and result in the production of a detectable fluorescent signal by the fluorescent donor moiety and fluorescent acceptor moiety at the wavelength of the fluorescent donor moiety. A compound that inhibits the activity of the tRNA splicing endonuclease will inhibit or reduce cleavage of the substrate and thus, increase the fluorescence emission of the fluorescent acceptor moiety at the wavelength of the fluorescent donor moiety. A compound that enhances the activity of the endogenous tRNA splicing endonuclease will enhance the cleavage of the substrate and thus, reduce the fluorescence emission of the fluorescent acceptor moiety at the wavelength of the fluorescent donor moiety.
[0028] Further, the effect of a compound on the activity of an animalia tRNA splicing endonuclease may be determined utilizing a tRNA endonuclease suppression
assay. In such an assay, a host cell is engineered to contain a
reporter gene and a
suppressor tRNA, wherein the
reporter gene construct comprises a
reporter gene with a nonsense codon in its
open reading frame such that the
open reading frame is interrupted and the
suppressor tRNA'
s expression is regulated by an inducible regulatory element and the
suppressor tRNA contains a tRNA
intron in the antisense codon; the expression of the suppressor tRNA is induced; the host cell is contacted with a compound; and the expression of the reporter
gene and / or the activity of the
protein encoded by the reporter
gene is measured utilizing techniques well-known to one of skill in the art or described herein. A compound that inhibits or reduces the activity of an animalia tRNA splicing endonuclease will inhibit or reduce the production of functional suppressor tRNA and thus, reduce the expression of the reporter
gene relative to a previously determined
reference range, or the expression of the reporter gene in the absence of the compound or the presence of an appropriate control (e.g., a
negative control). A compound that enhances the activity of an animalia tRNA splicing endonuclease will enhance the production of functional suppressor tRNA and thus, enhance the production of the reporter gene relative to a previously determined
reference range, or the expression of the reporter gene in the absence of the compound or the presence of an appropriate control (e.g., a
negative control).
[0046] As used herein, the term “effective amount” refers to the amount of a compound which is sufficient to reduce or ameliorate the progression, severity and / or duration of a proliferative disorder or one or more symptoms thereof, prevent the development, recurrence or onset of a proliferative disorder or one or more symptoms thereof, prevent the advancement of a proliferative disorder or one or more symptoms thereof, or enhance or improve the therapeutic(s) effect(s) of another therapy.
[0063] As used herein, the term “quencher” refers to a molecule or a part of a compound that is capable of reducing the emission from a fluorescent moiety. Such reduction includes reducing the light after the time when a
photon is normally emitted from a fluorescent moiety.
[0069] As used herein, the term “synergistic” refers to a combination of a compound identified using one of the methods described herein, and another therapy (e.g., agent) which has been or is currently being used to prevent, treat, manage or ameliorate a proliferative disorder or a symptom thereof, which is more effective than the additive effects of the therapies. A synergistic effect of a combination of therapies (e.g., prophylactic or therapeutic agents) permits the use of lower dosages of one or more of the therapies and / or less frequent administration of said therapies to a subject with a proliferative disorder. The ability to utilize lower dosages of a therapy (e.g., a prophylactic or therapeutic agent) and / or to administer said therapy less frequently reduces the
toxicity associated with the administration of said agent to a subject without reducing the
efficacy of said therapies in the prevention, treatment, management or amelioration of a proliferative disorder. In addition, a synergistic effect can result in improved
efficacy of therapies (e.g., agents) in the prevention, treatment, management or amelioration of a proliferative disorder. Finally, a synergistic effect of a combination of therapies (e.g., prophylactic or therapeutic agents) may avoid or reduce adverse or unwanted side effects associated with the use of either therapy alone.
 Login to View More
Login to View More