Polysaccharide sweetener compounds, process for manufacture, and method of selecting components for polysaccharide sweetener compounds based on user specific sweetener applications
a polysaccharide and sweetener technology, applied in the field of polysaccharide sweetener compounds, can solve the problems of high caloric content, hyperglycemic reaction, and does not possess other properties required to be fully functional, and achieves chemoselective promiscuous ligation, reduce pressure, and facilitate baric and/or thermal processing of said components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
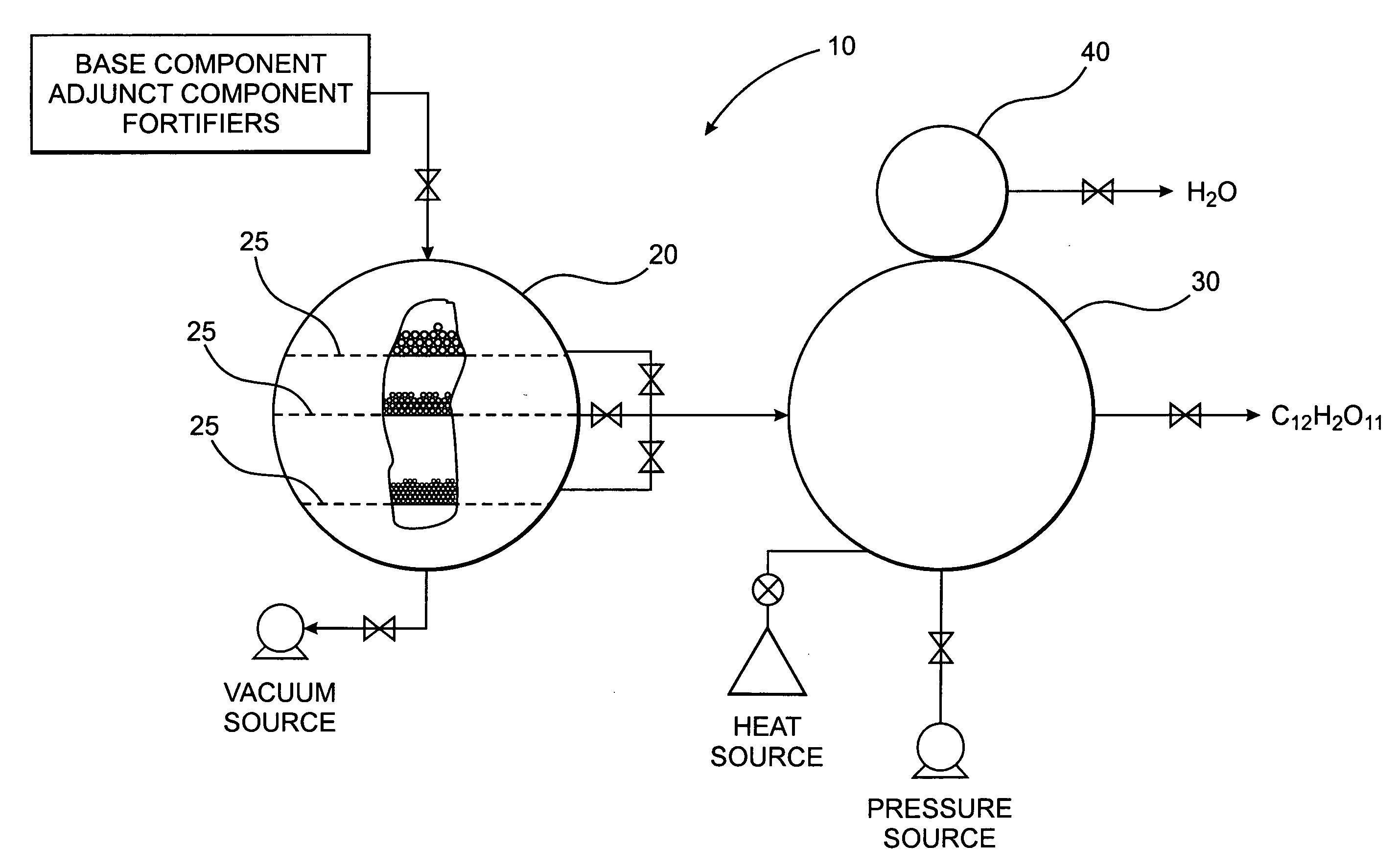
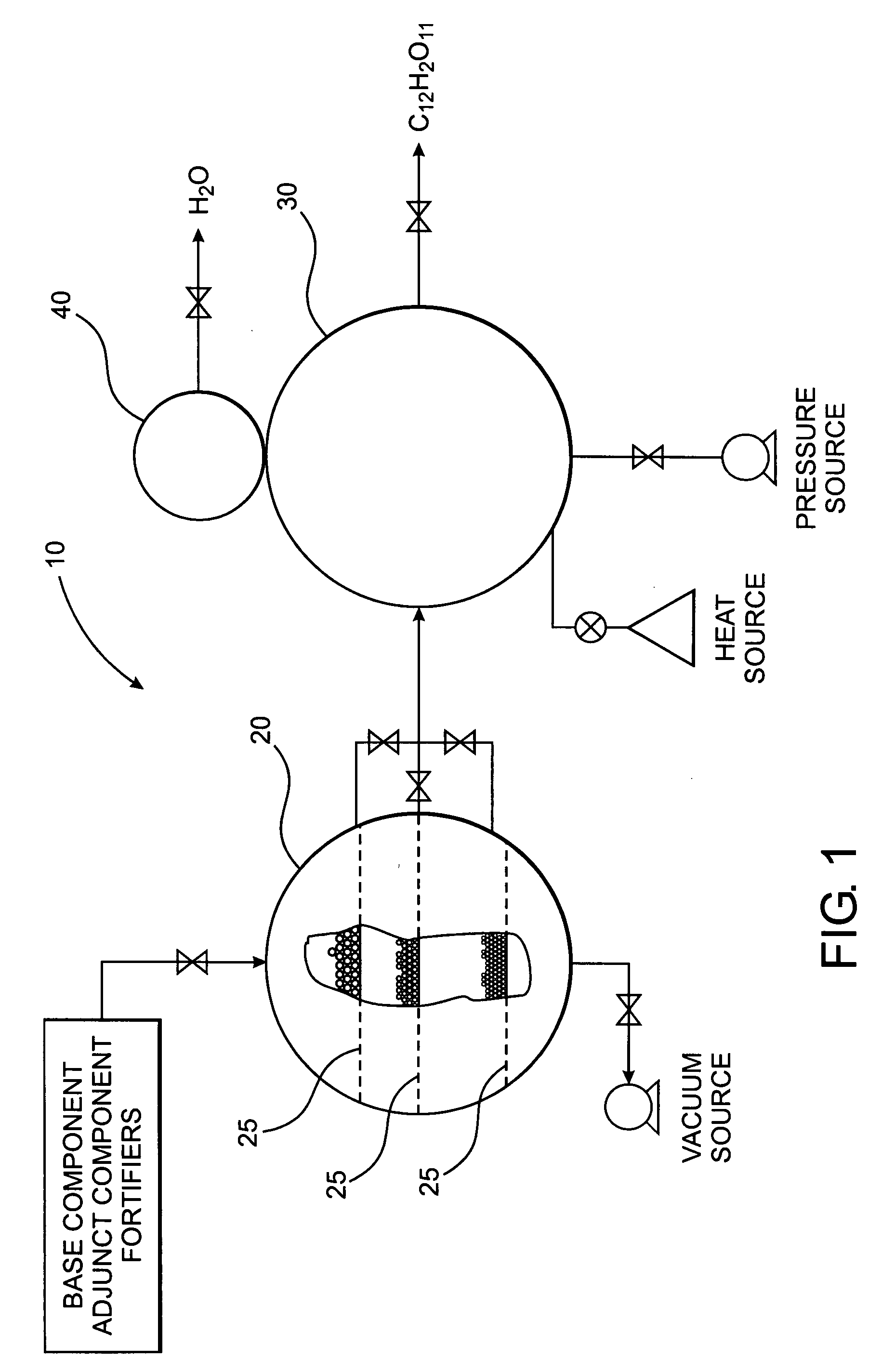
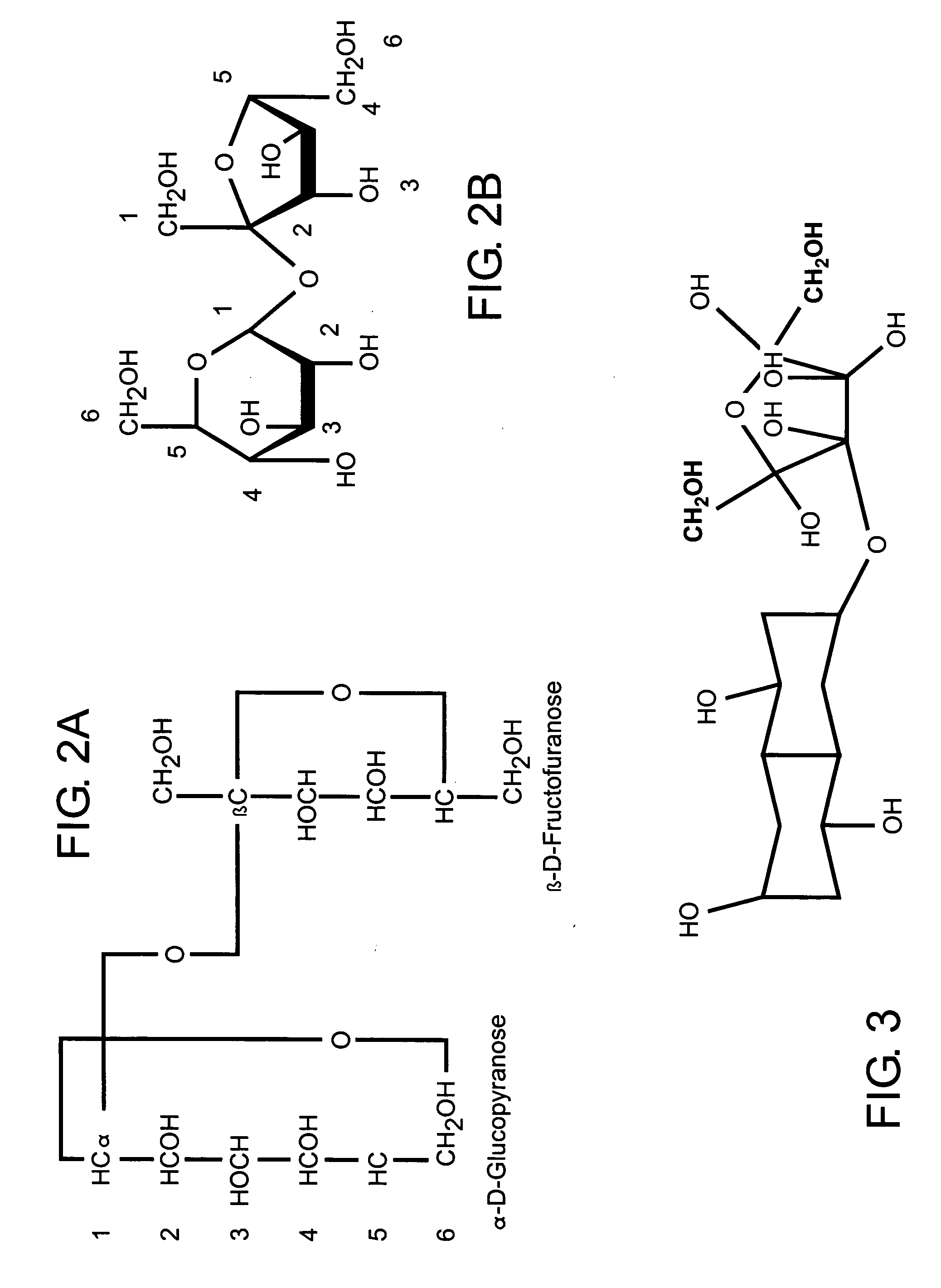
[0032] The present invention is directed to a novel polysaccharide composition derived from naturally occurring components which is functionally similar to sucrose, as illustrated in FIGS. 2A and 2B, or other natural sugar compounds and derivatives, without the negative aspects of such compounds. Specifically, the novel polysaccharide sweetener compound of the present invention is low in caloric content, and as they do not comprise glucose, they have a low-glycemic index and load so as to inhibit rapid elevation in blood sugar levels, while retaining the functionality and nutritive value of natural sweeteners in common use today. Further, as the novel polysaccharide sweetener compounds of the present invention are derived from naturally occurring components, they do not present the known and uncertain health risks associated with the various chemical sweeteners also currently in use today. In one preferred embodiment, the polysaccharide sweetener compound of the present invention co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com