Synthesis and anti-tumor activity of nitrogen substituted thalidomide analogs
a technology of thalidomide and analogues, which is applied in the field of synthesis and anti-tumor activity of nitrogen substituted thalidomide analogs, can solve the problems of increased thickness of lesion, increased risk of metastasis, and tumors progressing to large cavernous and infiltrative forms, so as to inhibit unwanted angiogenesis, inhibit angiogenesis, and be easily administered.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
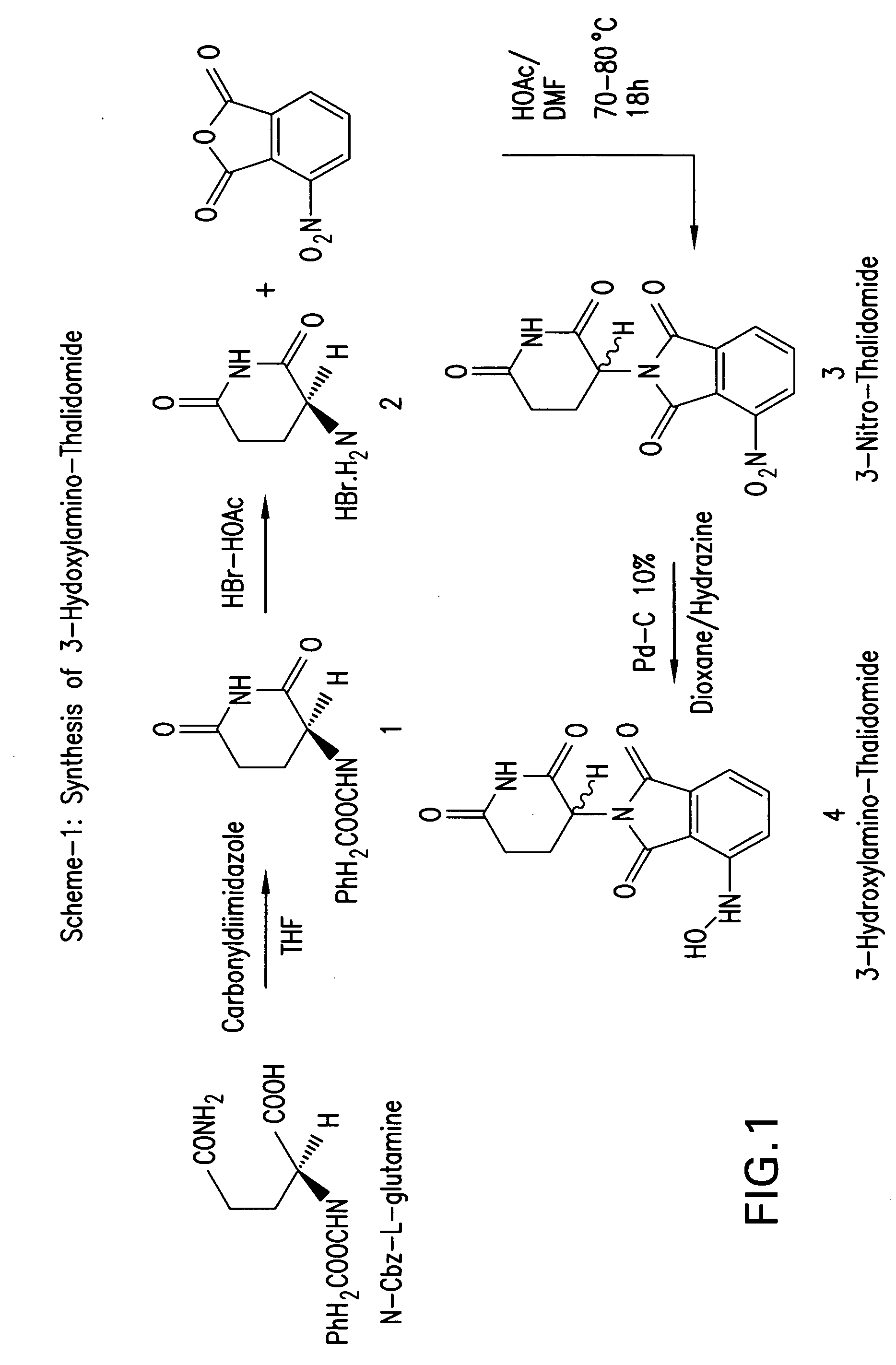
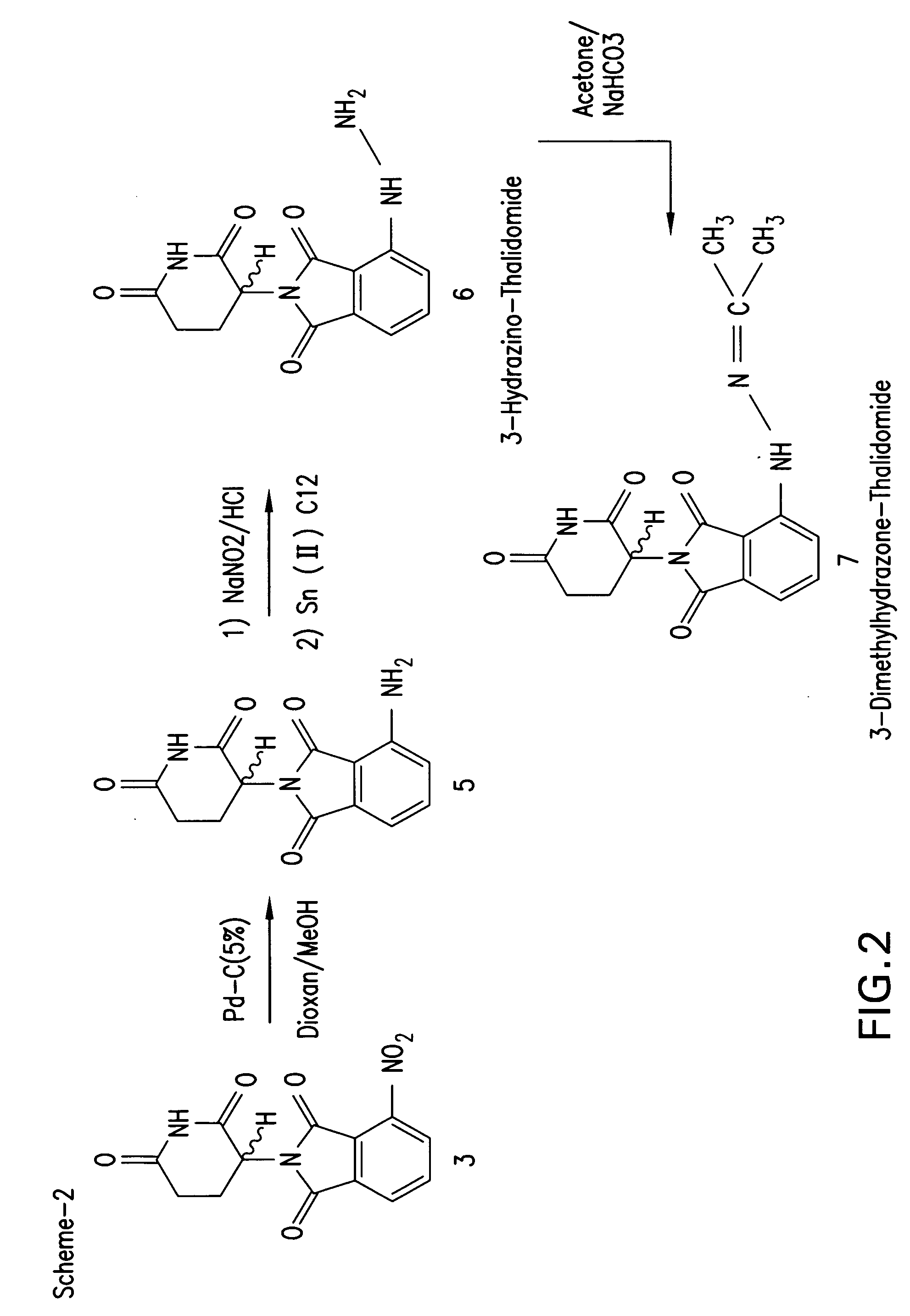
[0111] Synthesis of 3-hydazino-thalidomide and 3-hydoxylamino-thalidomide: 3-hydoxylamino-thalidomide 3-hydazino-thalidomide was synthesized as depicted in FIGS. 1 and 2.
[0112] First, N-carboxybenzyloxy-L-gluteramide (1) was synthesized. Synthesis of N-carboxybenzyloxy-L-gluteramide (1): Into a stirring solution of carboxybenzyloxy-L-glutamine (2.8 g, 10 mmols) in 40 mL THF anhydrous, 1,1-carbonyldiimidazole (1.92 g, 12 mmols) was added. (Alternatively, carboxybenzyloxy-L-glutamine can be cyclized by N,N-Dicyclohexylcabdiimide in THF or in dichloromethane to carboxybenzyloxy-L-gluteramide). The reaction mixture was heated under reflux for 18 hours. The THF was evaporated and the product was dissolved in chloroform. The chloroform layer was washed with water and brine and dried over CaSO4 anhydrous, filtered and evaporated to give white solid. The solid product was crystallized from ethyl ether to give 2.4 grams crystalline powder (90%). 1H NMR in CDC13 confirmed the product as carb...
example 2
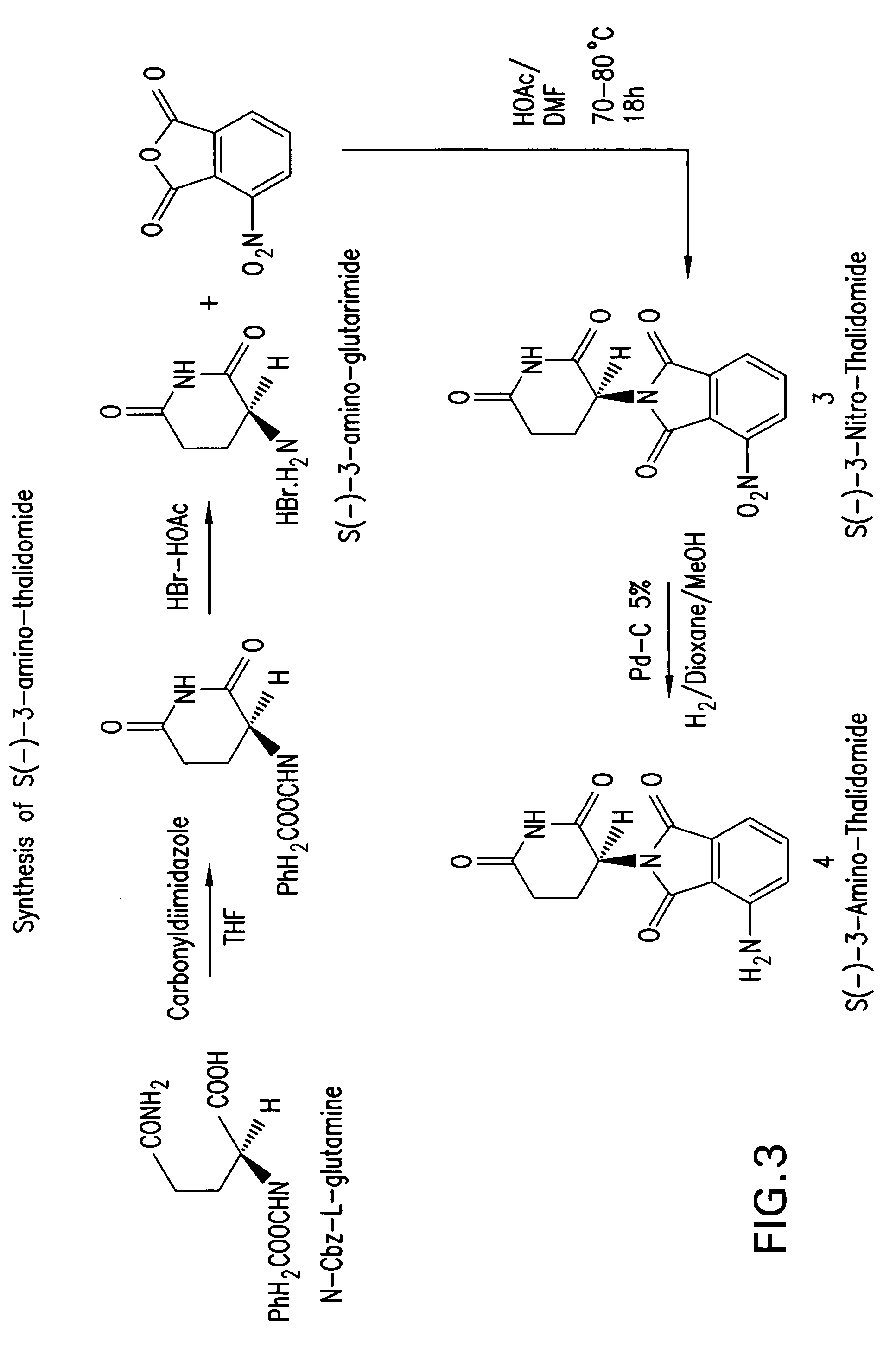
[0119] Synthesis of S-(−)-(3-benzyloxycarbonylamino)-glutarimide: Into a stirring solution of carboxybenzyloxy-L-glutamine (2.8 g, 10 mmols) in 40 mL THF anhydrous, 1,1-carbonyldiimidazole (1.92 g, 12 mmols) were added. The reaction mixture was heated under reflex for 18 hours. The THF was evaporated and the product was dissolved in chloroform. The chloroform layer was washed with water and brine and dried over CaSO4 anhydrous, filtered and evaporated to give white solid. The solid product was crystallized from ethyl ether to give 2.4 grams crystalline powder (90%). Alternatively, carboxybenzyloxy-L-glutamine can be cyclized by treating with SOC12 in DMF at about −70° C. to about 0° C. for 1 hour to S-(−)-(3-benzyloxycarbonylamino)-glutarimide. The reaction mixture was diluted with CHC13 and washed with 5% Na2C03, dried over Na2SO4 anhydrous, filtered, and evaporated to give 2.5 g (90%) S-(−)-(3-benzyloxycarbonylamino)-glutarimide). 1H NMR in CDC13 confirmed the product as S-(−)-(3-...
example 3
[0120] Synthesis of S-(−)-3-Amino-glutarimide.HBr: Into a solution of S-(−)-(3-benzyloxycarbonylamino)-glutarimide (1.2 g, 4.6 mmols) in 15 mL acetic acid glacial, 8 mL of 30% HBr / acetic acid solution was added at 20° C. The temperature of reaction mixture was raised to RT and stirred for 1 hour. White solid powder of S-(−)-2-Amino-gluteramide.HBr started appearing in the reaction mixture. The solid was filtered and washed with 5 mL acetic acid glacial and then with ether to give 1.8 g (80%) product. Analysis on polarimeter of product showed (−) rotation, [a]25D (c=1, water)=−37.50 and confirmed the product as S(−)-2-amino-gluteramide. 1H NMR in DMSO-D6 confirmed the product as 2-amino-L-gluteramide.HBr. 1H NMR (DMSO-D6, PPM), 11.60 (1H, s broad), 8.45 (3H, s broad), 4.4 (1H, dd, J=4.5, 3), 2.85-2.45 (2H, m), 2.25-1.90 (2H, m), m. p. 279-281° C. (lit=279° C.).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com