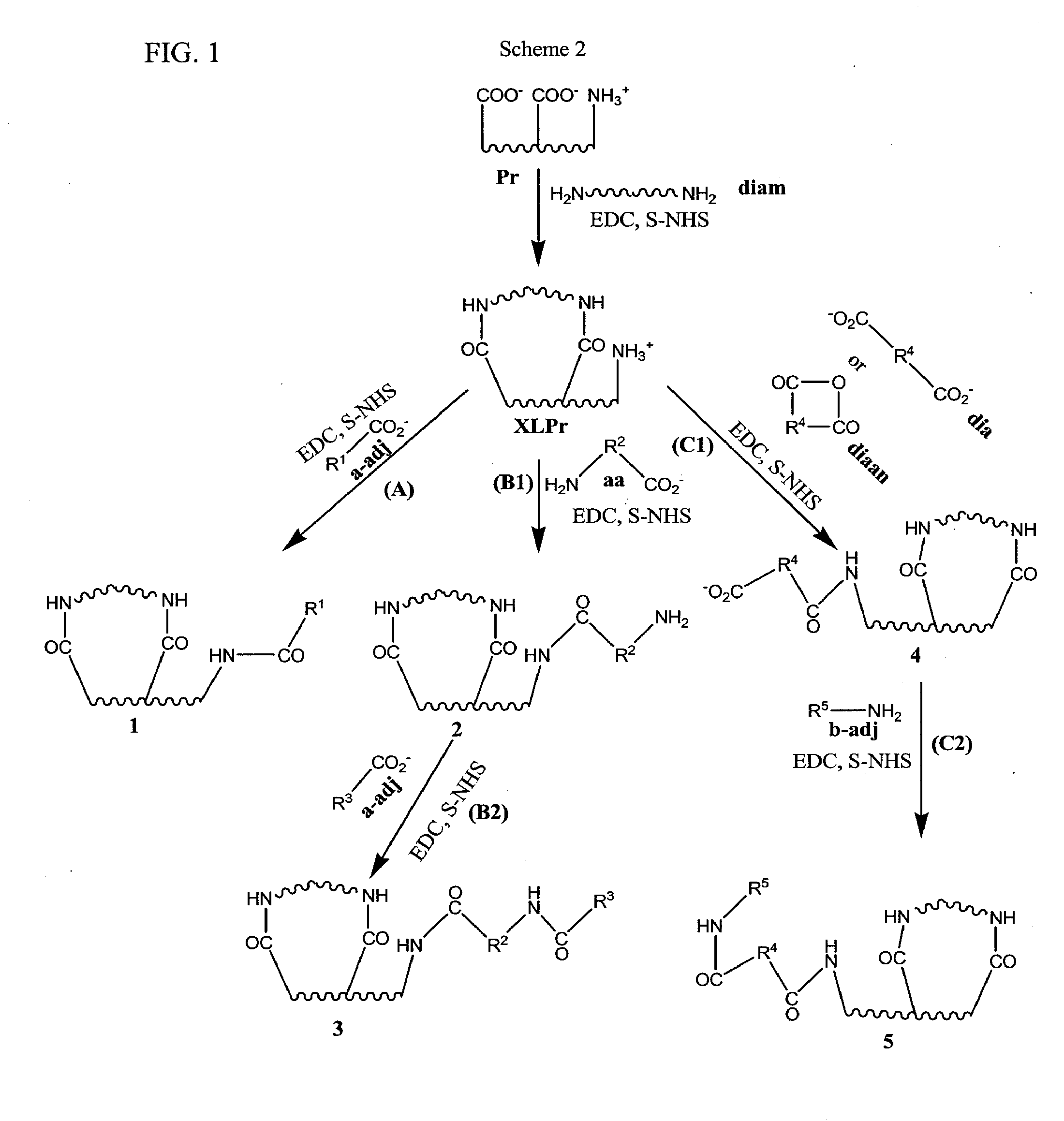
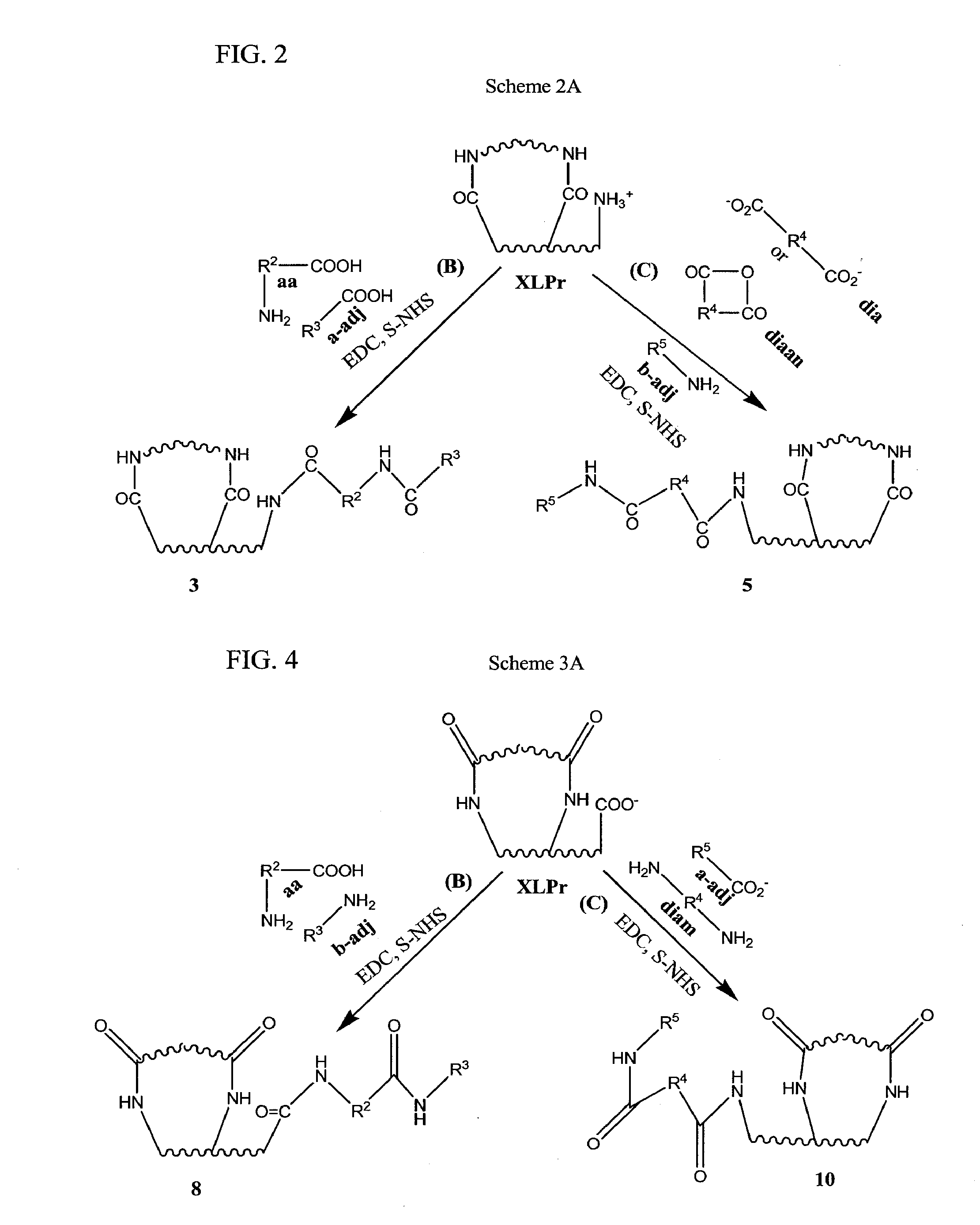
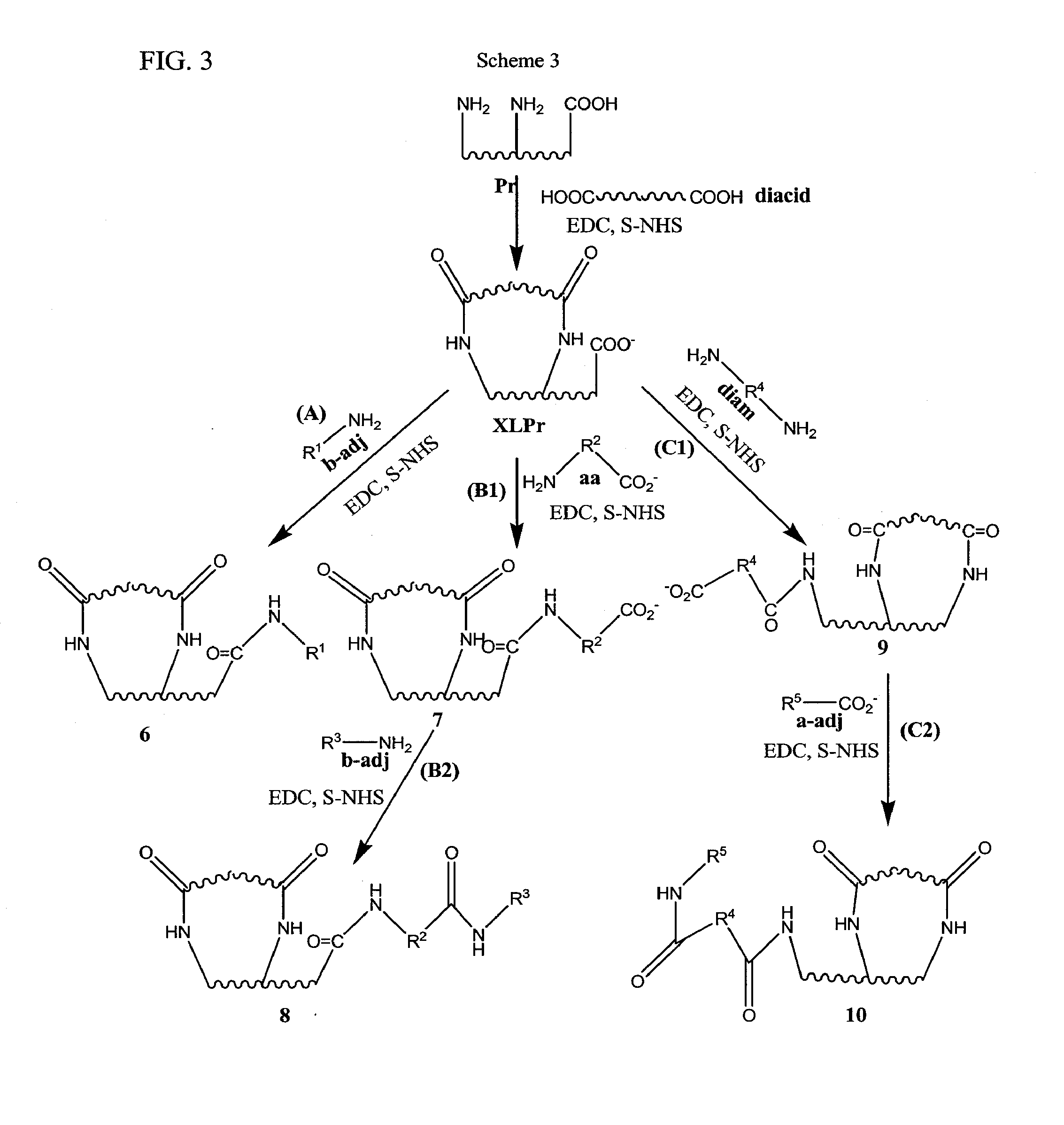
[0017]In some embodiments, the invention provides a process of making a bioimplant comprising a chemically sterilized biological tissue and at least one adjunct, wherein the adjunct is covalently conjugated to the biological tissue, comprising: (a) contacting a biological tissue with an adjunct to form a combination; and (b) contacting the combination with a chemical sterilizing agent to form the bioimplant. In some embodiments, the sterilizing agent is a carbodiimide, such as EDC, optionally in the presence of an alkanol, such as a C2-C4 alkanol, especially isopropanol. In some embodiments, the biological tissue comprises native tissue, processed tissue in native form, processed tissue in non-native form, a composite or a complex composite. In some embodiments: (1) said native tissue comprises bone, tendon, ligament, dermis, fascia, pericardium, said native tissue comprises bone, tendon, ligament, dermis, fascia, pericardium, and combinations thereof, including bone-connective tissue combinations, such as bone-tendon combinations and bone-ligament-bone combinations; (2) said processed tissue in native form comprises crosslinked tissue, decellularized crushed bone fragments, decellularized collagen or other decellularized and/or defatted bone, tendon, ligament, fascia or bone-connective tissue combinations, such as bone-ligament-bone or bone-tendon combinations; (3) said processed tissue in non-native form comprises solubilized or purified collagen from connective tissue, gelatin from mammals or fish or demineralized bone; (4) said composites comprise combinations of native tissues, processed tissues in native form and/or processed tissues in non-native form, such as pericardium with gelatin, bone with gelatin, purified collagen with gelatin or demineralized bone with solubilized or purified collagen; and (5) said complex composite comprises native tissue, processed tissue in native form, processed tissue in non-native form or a composite of native tissue, processed tissue in native form and/or processed tissue in non-native form with a biocompatible material such as a hydrogel, an alginate and/or chitosan. In some embodiments, the adjunct is a protein, a small peptide, a ribonucleic acid, a deoxyribonucleic acid, a polysaccharide, glycosaminoglycan (GAG) or an antibiotic. In some embodiments, the adjunct comprises: (1) one or more proteoglycans or glycosaminoglycans, (2) one or more proteins, such as: (a) any member of the Transforming Growth Factor (TGF) superfamily, such as BMP-2, BMP-4 and BMP-7, transforming growth factor-β (TGF-β; (b) platelet derived growth factor (PDGF); (c) fibroblast growth factor (FGF); (d) insulin-like growth factors (IGF); (e) cartilage-derived growth factors (CDGF); (3) a deoxyribonucleic acid selected from genes, gene fragments and antisense DNA; (4) ribonucleic acid such as a small interfering RNA (siRNA) or a microRNA; or (5) an antibiotic, such as one or more aminoglycosides, amphenicols, ansamycins, β-lactams, lincosamides, macrolides, polypeptide antibiotics, tetracyclines, cycloserine, mupirocin, tuberin, 2,4-diaminopyrimidines, nitrofurans, quinolones, sulfonamides, sulfones, clofoctol, hexedine, methenamine, nitroxoline, taurolidine and xibernol. In some embodiments, the adjunct is (1) one or more proteoglycans or glycosaminoglycans, (2) one or more proteins, such as: (a) any member of the Transforming Growth Factor (TGF) superfamily, such as BMP-2, BMP-4 and BMP-7, transforming growth factor-β (TGF-β); (b) platelet derived growth factor (PDGF); (c) fibroblast growth factor (FGF); (d) insulin-like growth factors (IGF); (e) cartilage-derived growth factors (CDGF); (3) a deoxyribonucleic acid selected from genes, gene fragments and antisense DNA; (3) an antibiotic, such as one or more aminoglycosides, amphenicols, ansamycins, β-lactams, lincosamides, macrolides, polypeptide antibiotics, tetracyclines, cycloserine, mupirocin, tuberin, 2,4-diaminopyrimidines, nitrofurans, quinolones, sulfonamides, sulfones, clofoctol, hexedine, methenamine, nitroxoline, taurolidine and xibemol. In some embodiments, the biological tissue comprises collagen, purified collagen or solubilized collagen. In some embodiments, the method includes shaping or forming the biological tissue into the form of a suture, a sheet, an implantable valve, an implantable sponge or an implantable paste. In some embodiments, the invention provides a bioimplant made by the process comprising contacting a biological tissue with an adjunct molecule in the presence of a sterilizing agent, as described herein. In some embodiments, the adjunct retains at least some of its native activity after it has been conjugated to the biological tissue. In some embodiments, the adjunct is adapted to be released in vivo and the adjunct, once release in vivo possesses at least some of its native activity.
[0018]In some embodiments, the invention provides a process of making a sterilized biological implant, comprising: (a) contacting a starting tissue with an adjunct to form an intermediate; (b) freezing the intermediate product of (a) to produce a frozen intermediate; (c) lyophilizing the frozen intermediate from (b) to produce a lyophilized intermediate; and (d) contacting the lyophilized intermediate with a sterilizing solution comprising a carbodiimide sterilizing agent to produce the biological implant. In some embodiments, the sterilizing agent is a carbodiimide, such as EDC, optionally in the presence of an alkanol, such as a C2-C4 alkanol, especially isopropanol. In some embodiments, the starting tissue is a native tissue, a processed tissue in native form or a composite tissue. In some embodiments, the starting tissue is: (1) a native tissue comprising bone, tendon, ligament, dermis, fascia, pericardium, and combinations thereof, including bone-connective tissue combinations, such as bone-tendon combinations and bone-ligament-bone combinations; (2) said processed tissue in native form comprises crosslinked tissue, decellularized crushed bone fragments, decellularized collagen or other decellularized and/or defatted bone, tendon, ligament, fascia or bone-connective tissue combinations, such as bone-ligament-bone or bone-tendon combinations; or (3) a composite comprising combinations of native tissues, processed tissues in native form and/or processed tissues in non-native form, such as pericardium with gelatin, bone with gelatin, purified collagen with gelatin or demineralized bone with solubilized or purified collagen. In some embodiments, the adjunct is a protein, a small peptide, a ribonucleic acid, a deoxyribonucleic acid, a polysaccharide, glycosaminoglycan (GAG) or an antibiotic. In some embodiments, the adjunct comprises: (1) one or more proteoglycans or glycosaminoglycans, (2) one or more proteins, such as: (a) any member of the Transforming Growth Factor (TGF) superfamily, such as BMP-2, BMP-4 and BMP-7, transforming growth factor-β (TGF-β; (b) platelet derived growth factor (PDGF); (c) fibroblast growth factor (FGF); (d) insulin-like growth factors (IGF); (e) cartilage-derived growth factors (CDGF); (3) a deoxyribonucleic acid selected from genes, gene fragments and antisense DNA; (4) ribonucleic acid such as a small interfering RNA (siRNA) or a microRNA; or (5) an antibiotic, such as one or more aminoglycosides, amphenicols, ansamycins, β-lactams, lincosamides, macrolides, polypeptide antibiotics, tetracyclines, cycloserine, mupirocin, tuberin, 2,4-diaminopyrimidines, nitrofurans, quinolones, sulfonamides, sulfones, clofoctol, hexedine, methenamine, nitroxoline, taurolidine and xibemol. In some embodiments, the adjunct is (1) one or more proteoglycans or glycosaminoglycans, (2) one or more proteins, such as: (a) any member of the Transforming Growth Factor (TGF) superfamily, such as BMP-2, BMP-4 and BMP-7, transforming growth factor-β (TGF-β); (b) platelet derived growth factor (PDGF); (c) fibroblast growth factor (FGF); (d) insulin-like growth factors (IGF); (e) cartilage-derived growth factors (CDGF); (3) an antibiotic, such as one or more aminoglycosides, amphenicols, ansamycins, β-lactams, lincosamides, macrolides, polypeptide antibiotics, tetracyclines, cycloserine, mupirocin, tuberin, 2,4-diaminopyrimidines, nitrofurans, quinolones, sulfonamides, sulfones, clofoctol, hexedine, methenamine, nitroxoline, taurolidine and xibernol. In some embodiments, the biological tissue comprises collagen, purified collagen or solubilized collagen. In some embodiments, the method includes shaping or forming the biological tissue into the form of a suture, a sheet, an implantable valve, an implantable sponge or an implantable paste. In some embodiments, the invention provides a bioimplant produced by the foregoing methods. In some embodiments, the adjunct retains at least some of its native activity after it has been conjugated to the biological tissue. In some embodiments, the adjunct is adapted to be released in vivo and the adjunct, once release in vivo possesses at least some of its native activity.
[0019]In some embodiments, the invention provides a process of making a sterilized biological implant, comprising: (a) preparing a composition comprising a starting tissue; (b) freezing the composition from (a) to form a frozen composition; (c) lyophilizing the frozen composition from (b) to form a lyophilized composition; and (d) contacting the lyophilized composition from (c) with a sterilizing solution comprising a sterilizing agent and an adjunct to produce the biological implant. In some embodiments, the sterilizing agent is a carbodiimide, such as EDC, optionally in the presence of an alkanol, such as a C2-C4 alkanol, especially isopropanol. In some embodiments, the starting tissue is a processed tissue in native form or a complex composite. In some embodiments, the starting tissue is: (1) a processed tissue in native form comprising crosslinked tissue, decellularized crushed bone fragments, decellularized collagen or other decellularized and/or defatted bone, tendon, ligament, fascia or bone-connective tissue combinations, such as bone-ligament-bone or bone-tendon combinations; or (2) complex composite comprising native tissue, processed tissue in native form, processed tissue in non-native form or a composite of native tissue, processed tissue in native form and/or processed tissue in non-native form with a biocompatible material such as a hydrogel, an alginate and/or chitosan. In some embodiments, the adjunct is a protein, a small peptide, a ribonucleic acid, a deoxyribonucleic acid, a polysaccharide, glycosaminoglycan (GAG) or an antibiotic. In some embodiments, the adjunct comprises: (1) one or more proteoglycans or glycosaminoglycans, (2) one or more proteins, such as: (a) any member of the Transforming Growth Factor (TGF) superfamily, such as BMP-2, BMP-4 and BMP-7, transforming growth factor-β (TGF-β); (b) platelet derived growth factor (PDGF); (c) fibroblast growth factor (FGF); (d) insulin-like growth factors (IGF); (e) cartilage-derived growth factors (CDGF); (3) a deoxyribonucleic acid selected from genes, gene fragments and antisense DNA; (4) ribonucleic acid such as a small interfering RNA (siRNA) or a microRNA; or (5) an antibiotic, such as one or more aminoglycosides, amphenicols, ansamycins, β-lactams, lincosamides, macrolides, polypeptide antibiotics, tetracyclines, cycloserine, mupirocin, tuberin, 2,4-diaminopyrimidines, nitrofurans, quinolones, sulfonamides, sulfones, clofoctol, hexedine, methenamine, nitroxoline, taurolidine and xibemol. In some embodiments, the adjunct is (1) one or more proteoglycans or glycosaminoglycans, (2) one or more proteins, such as: (a) any member of the Transforming Growth Factor (TGF) superfamily, such as BMP-2, BMP-4 and BMP-7, transforming growth factor-β (TGF-β); (b) platelet derived growth factor (PDGF); (c) fibroblast growth factor (FGF); (d) insulin-like growth factors (IGF); (e) cartilage-derived growth factors (CDGF); (3) a deoxyribonucleic acid selected from genes, gene fragments and antisense DNA; (3) an antibiotic, such as one or more aminoglycosides, amphenicols, ansamycins, β-lactams, lincosamides, macrolides, polypeptide antibiotics, tetracyclines, cycloserine, mupirocin, tuberin, 2,4-diaminopyrimidines, nitrofurans, quinolon
 Login to View More
Login to View More